Yonsei Med J.
2017 May;58(3):619-625. 10.3349/ymj.2017.58.3.619.
Gender-Specific Associations between CHGB Genetic Variants and Schizophrenia in a Korean Population
- Affiliations
-
- 1Department of Life Science, Sogang University, Seoul, Korea. hdshin@sogang.ac.kr
- 2Research Institute for Basic Science, Sogang University, Seoul, Korea.
- 3Department of Psychiatry, College of Medicine, Gyeongsang National University, Jinju, Korea.
- 4Division of Life Science, Research Institute of Life Science, Gyeongsang National University, Jinju, Korea.
- 5Department of Neuropsychiatry, Hallym University Hangang Sacred Heart Hospital, Seoul, Korea.
- 6Department of Neuropsychiatry, Soonchunhyang University Hospital, Seoul, Korea. siwoo@schmc.ac.kr
- 7Department of Genetic Epidemiology, SNP Genetics, Inc., Seoul, Korea.
- KMID: 2419121
- DOI: http://doi.org/10.3349/ymj.2017.58.3.619
Abstract
- PURPOSE
Schizophrenia is a devastating mental disorder and is known to be affected by genetic factors. The chromogranin B (CHGB), a member of the chromogranin gene family, has been proposed as a candidate gene associated with the risk of schizophrenia. The secretory pathway for peptide hormones and neuropeptides in the brain is regulated by chromogranin proteins. The aim of this study was to investigate the potential associations between genetic variants of CHGB and schizophrenia susceptibility.
MATERIALS AND METHODS
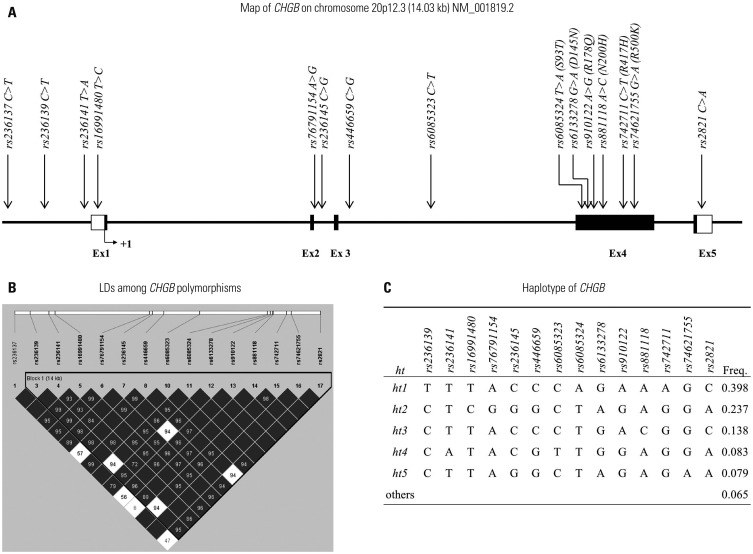
In the current study, 15 single nucleotide polymorphisms of CHGB were genotyped in 310 schizophrenia patients and 604 healthy controls.
RESULTS
Statistical analysis revealed that two genetic variants (non-synonymous rs910122; rs2821 in 3"²-untranslated region) were associated with schizophrenia [minimum p=0.002; odds ratio (OR)=0.72], even after correction for multiple testing (p(corr)=0.02). Since schizophrenia is known to be differentially expressed between sexes, additional analysis for sex was performed. As a result, these two genetic variants (rs910122 and rs2821) and a haplotype (ht3) showed significant associations with schizophrenia in male subjects (p(corr)=0.02; OR=0.64), whereas the significance disappeared in female subjects (p>0.05).
CONCLUSION
Although this study has limitations including a small number of samples and lack of functional study, our results suggest that genetic variants of CHGB may have sex-specific effects on the risk of schizophrenia and provide useful preliminary information for further study.
MeSH Terms
-
Adult
Asian Continental Ancestry Group/*genetics
Brain/*metabolism/physiopathology
Case-Control Studies
Chromogranin B/*genetics
Female
Genetic Markers/genetics
Genetic Predisposition to Disease/*genetics
Genotype
Haplotypes
Humans
Male
Middle Aged
Polymorphism, Single Nucleotide/*genetics
Republic of Korea/epidemiology
Schizophrenia/ethnology/*genetics
Sex Factors
Chromogranin B
Genetic Markers
Figure
Reference
-
2. Aleman A, Kahn RS, Selten JP. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch Gen Psychiatry. 2003; 60:565–571. PMID: 12796219.3. Ochoa S, Usall J, Cobo J, Labad X, Kulkarni J. Gender differences in schizophrenia and first-episode psychosis: a comprehensive literature review. Schizophr Res Treatment. 2012; 2012:916198. PMID: 22966451.
Article5. Owen MJ, Craddock N, O'Donovan MC. Schizophrenia: genes at last? Trends Genet. 2005; 21:518–525. PMID: 16009449.
Article6. Chen XW, Feng YQ, Hao CJ, Guo XL, He X, Zhou ZY, et al. DTNBP1, a schizophrenia susceptibility gene, affects kinetics of transmitter release. J Cell Biol. 2008; 181:791–801. PMID: 18504299.
Article7. Gamo NJ, Duque A, Paspalas CD, Kata A, Fine R, Boven L, et al. Role of disrupted in schizophrenia 1 (DISC1) in stress-induced prefrontal cognitive dysfunction. Transl Psychiatry. 2013; 3:e328. PMID: 24301646.
Article8. Harrison PJ, Law AJ. Neuregulin 1 and schizophrenia: genetics, gene expression, and neurobiology. Biol Psychiatry. 2006; 60:132–140. PMID: 16442083.
Article9. Iijima Y, Inada T, Ohtsuki T, Senoo H, Nakatani M, Arinami T. Association between chromogranin B gene polymorphisms and schizophrenia in the Japanese population. Biol Psychiatry. 2004; 56:10–17. PMID: 15219467.
Article10. Wu S, Ma J, Xing Q, Xu Y, Meng J, Cao D, et al. Further evidence that the chromogranin B gene confers predisposition to schizophrenia: a family-based association study in Chinese. J Neural Transm (Vienna). 2007; 114:641–644. PMID: 17143778.
Article11. Kitao Y, Inada T, Arinami T, Hirotsu C, Aoki S, Iijima Y, et al. A contribution to genome-wide association studies: search for susceptibility loci for schizophrenia using DNA microsatellite markers on chromosomes 19, 20, 21 and 22. Psychiatr Genet. 2000; 10:139–143. PMID: 11204350.
Article12. Nowakowski C, Kaufmann WA, Adlassnig C, Maier H, Salimi K, Jellinger KA, et al. Reduction of chromogranin B-like immunore-activity in distinct subregions of the hippocampus from individuals with schizophrenia. Schizophr Res. 2002; 58:43–53. PMID: 12363389.
Article13. Zhang B, Tan Z, Zhang C, Shi Y, Lin Z, Gu N, et al. Polymorphisms of chromogranin B gene associated with schizophrenia in Chinese Han population. Neurosci Lett. 2002; 323:229–233. PMID: 11959426.
Article14. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. Washington, DC: American Psychiatric Association;1994.15. Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005; 21:263–265. PMID: 15297300.
Article16. Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001; 68:978–989. PMID: 11254454.
Article17. Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet. 2004; 74:765–769. PMID: 14997420.
Article18. Seeman P, Kapur S. Schizophrenia: more dopamine, more D2 receptors. Proc Natl Acad Sci U S A. 2000; 97:7673–7675. PMID: 10884398.
Article19. Zheng W, Wang H, Zeng Z, Lin J, Little PJ, Srivastava LK, et al. The possible role of the Akt signaling pathway in schizophrenia. Brain Res. 2012; 1470:145–158. PMID: 22771711.
Article20. Landén M, Grenfeldt B, Davidsson P, Stridsberg M, Regland B, Gottfries CG, et al. Reduction of chromogranin A and B but not C in the cerebrospinal fluid in subjects with schizophrenia. Eur Neuropsychopharmacol. 1999; 9:311–315. PMID: 10422891.
Article21. Lechner T, Adlassnig C, Humpel C, Kaufmann WA, Maier H, Reinstadler-Kramer K, et al. Chromogranin peptides in Alzheimer's disease. Exp Gerontol. 2004; 39:101–113. PMID: 14724070.
Article22. Nilsson A, Fälth M, Zhang X, Kultima K, Sköld K, Svenningsson P, et al. Striatal alterations of secretogranin-1, somatostatin, prodynorphin, and cholecystokinin peptides in an experimental mouse model of Parkinson disease. Mol Cell Proteomics. 2009; 8:1094–1104. PMID: 19131325.
Article23. Zhang K, Biswas N, Gayen JR, Miramontes-Gonzalez JP, Hightower CM, Mustapic M, et al. Chromogranin B: intra- and extra-cellular mechanisms to regulate catecholamine storage and release, in catecholaminergic cells and organisms. J Neurochem. 2014; 129:48–59. PMID: 24266713.
Article24. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014; 511:421–427. PMID: 25056061.25. Maric NP, Svrakic DM. Why schizophrenia genetics needs epigenetics: a review. Psychiatr Danub. 2012; 24:2–18. PMID: 22447077.26. Taupenot L, Harper KL, O'Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003; 348:1134–1149. PMID: 12646671.
Article27. Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010; 67:231–239. PMID: 20194823.
Article28. Saria A, Troger J, Kirchmair R, Fischer-Colbrie R, Hogue-Angeletti R, Winkler H. Secretoneurin releases dopamine from rat striatal slices: a biological effect of a peptide derived from secretogranin II (chromogranin C). Neuroscience. 1993; 54:1–4. PMID: 8515836.
Article29. Ren J, Jiang C, Gao X, Liu Z, Yuan Z, Jin C, et al. PhosSNP for systematic analysis of genetic polymorphisms that influence protein phosphorylation. Mol Cell Proteomics. 2010; 9:623–634. PMID: 19995808.
Article30. Savas S, Ozcelik H. Phosphorylation states of cell cycle and DNA repair proteins can be altered by the nsSNPs. BMC Cancer. 2005; 5:107. PMID: 16111488.
Article31. Ryu GM, Song P, Kim KW, Oh KS, Park KJ, Kim JH. Genome-wide analysis to predict protein sequence variations that change phosphorylation sites or their corresponding kinases. Nucleic Acids Res. 2009; 37:1297–1307. PMID: 19139070.
Article32. Langouët M, Saadi A, Rieunier G, Moutton S, Siquier-Pernet K, Fernet M, et al. Mutation in TTI2 reveals a role for triple T complex in human brain development. Hum Mutat. 2013; 34:1472–1476. PMID: 23956177.
Article33. Chaudhary MW, Al-Baradie RS. Ataxia-telangiectasia: future prospects. Appl Clin Genet. 2014; 7:159–167. PMID: 25258552.34. Gururajan A, van den Buuse M. Is the mTOR-signalling cascade disrupted in Schizophrenia? J Neurochem. 2014; 129:377–387. PMID: 24266366.
Article35. Jaros JA, Martins-de-Souza D, Rahmoune H, Rothermundt M, Leweke FM, Guest PC, et al. Protein phosphorylation patterns in serum from schizophrenia patients and healthy controls. J Proteomics. 2012; 76 Spec No.:43–55. PMID: 22641159.
Article36. Canuso CM, Pandina G. Gender and schizophrenia. Psychopharmacol Bull. 2007; 40:178–190. PMID: 18227787.37. Ram Murthy A, Purushottam M, Kiran Kumar HB, ValliKiran M, Krishna N, Jayramu Sriharsha K, et al. Gender-specific association of TSNAX/DISC1 locus for schizophrenia and bipolar affective disorder in South Indian population. J Hum Genet. 2012; 57:523–530. PMID: 22673686.
Article38. Mukai J, Liu H, Burt RA, Swor DE, Lai WS, Karayiorgou M, et al. Evidence that the gene encoding ZDHHC8 contributes to the risk of schizophrenia. Nat Genet. 2004; 36:725–731. PMID: 15184899.
Article39. Zhang K, Rao F, Rana BK, Gayen JR, Calegari F, King A, et al. Autonomic function in hypertension; role of genetic variation at the catecholamine storage vesicle protein chromogranin B. Circ Cardiovasc Genet. 2009; 2:46–56. PMID: 20011129.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Association Analysis between Chromogranin B Genetic Variations and Smooth Pursuit Eye Movement Abnormality in Korean Patients with Schizophrenia
- Association Study between Norepinephrine Transporter Gene Polymorphism and Schizophrenia in a Korean Population
- Comparison of Polygenic Risk for Schizophrenia between European and Korean Populations
- Gender-specific Associations of the Brain-derived Neurotrophic Factor Val66Met Polymorphism with Neurocognitive and Clinical Features in Schizophrenia
- Associations of Ubiquitin-Specific Protease Genes with Resilience and Social Anxiety in Healthy Youths