Korean J Physiol Pharmacol.
2018 Sep;22(5):525-538. 10.4196/kjpp.2018.22.5.525.
Tetramethylpyrazine reverses anxiety-like behaviors in a rat model of post-traumatic stress disorder
- Affiliations
-
- 1Acupuncture and Meridian Science Research Center, Kyung Hee University, Seoul 02447, Korea. bombi@khu.ac.kr
- 2Center for Converging Humanities, Kyung Hee University, Seoul 02447, Korea.
- 3Department of Physiology, College of Medicine, Kyung Hee University, Seoul 02447, Korea. dhhahm@khu.ac.kr
- KMID: 2419002
- DOI: http://doi.org/10.4196/kjpp.2018.22.5.525
Abstract
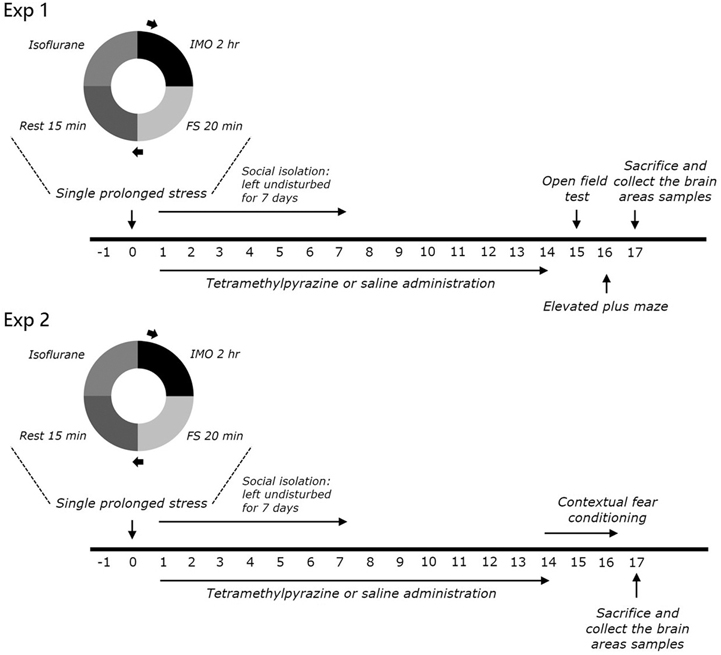
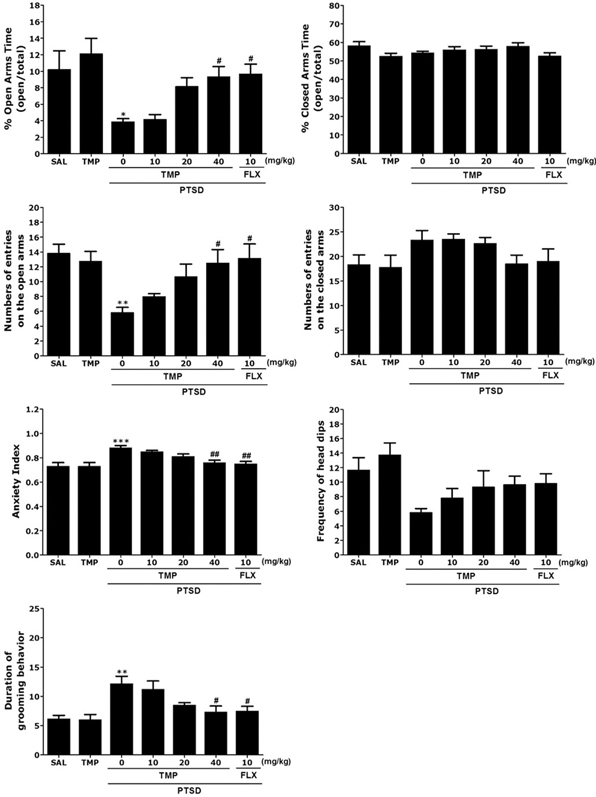
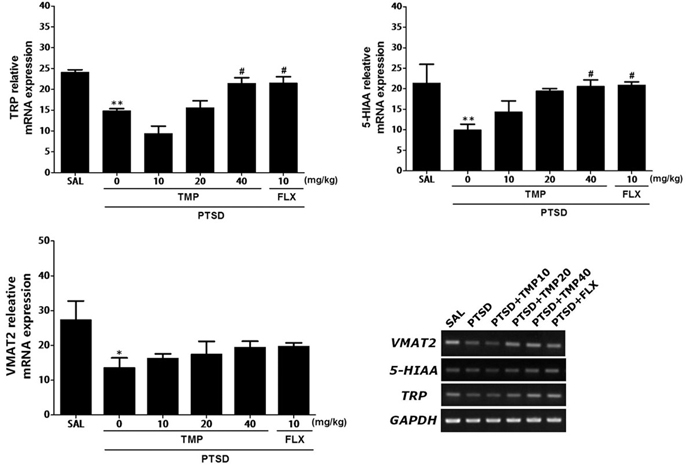
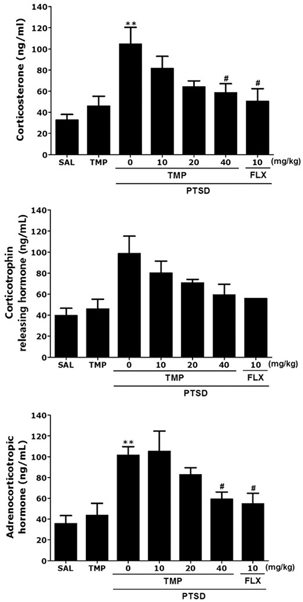
- Post-traumatic stress disorder (PTSD) is a trauma-induced psychiatric disorder characterized by impaired fear extermination, hyperarousal, and anxiety that may involve the release of monoamines in the fear circuit. The reported pharmacological properties of tetramethylpyrazine (TMP) include anti-cancer, anti-diabetic, anti-atherosclerotic, and neuropsychiatric activities. However, the anxiolytic-like effects of TMP and its mechanism of action in PTSD are unclear. This study measured several anxiety-related behavioral responses to examine the effects of TMP on symptoms of anxiety in rats after single prolonged stress (SPS) exposure by reversing the serotonin (5-HT) and hypothalamic-pituitary-adrenal (HPA) axis dysfunction. Rats were given TMP (10, 20, or 40 mg/kg, i.p.) for 14 days after SPS exposure. Administration of TMP significantly reduced grooming behavior, increased the time spent and number of visits to the open arm in the elevated plus maze test, and significantly increased the number of central zone crossings in the open field test. TMP administration significantly reduced the freezing response to contextual fear conditioning and significantly restored the neurochemical abnormalities and the SPS-induced decrease in 5-HT tissue levels in the prefrontal cortex and hippocampus. The increased 5-HT concentration during TMP treatment might be partially attribute to the tryptophan and 5-hydroxyindoleacetic acid mRNA level expression in the hippocampus of rats with PTSD. These findings support a role for reducing the altered serotonergic transmission in rats with PTSD. TMP simultaneously attenuated the HPA axis dysfunction. Therefore, TMP may be useful for developing an agent for treating psychiatric disorders, such those observed in patients with PTSD.
Keyword
MeSH Terms
Figure
Reference
-
1. Enman NM, Arthur K, Ward SJ, Perrine SA, Unterwald EM. Anhedonia, reduced cocaine reward, and dopamine dysfunction in a rat model of posttraumatic stress disorder. Biol Psychiatry. 2015; 78:871–879.
Article2. Wilson CB, McLaughlin LD, Ebenezer PJ, Nair AR, Francis J. Valproic acid effects in the hippocampus and prefrontal cortex in an animal model of post-traumatic stress disorder. Behav Brain Res. 2014; 268:72–80.
Article3. Ji LL, Tong L, Xu BK, Fu CH, Shu W, Peng JB, Wang ZY. Intra-hip pocampal administration of ZIP alleviates depressive and anxiety-like responses in an animal model of posttraumatic stress disorder. Behav Brain Funct. 2014; 10:28.4. Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev. 2005; 29:1207–1223.
Article5. Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010; 35:169–191.
Article6. Wu ZM, Ni GL, Shao AM, Cui R. Genistein alleviates anxiety-like behaviors in post-traumatic stress disorder model through enhancing serotonergic transmission in the amygdala. Psychiatry Res. 2017; 255:287–291.
Article7. Lin CC, Tung CS, Liu YP. Escitalopram reversed the traumatic stress-induced depressed and anxiety-like symptoms but not the deficits of fear memory. Psychopharmacology (Berl). 2016; 233:1135–1146.
Article8. George SA, Stout SA, Tan M, Knox D, Liberzon I. Early handling attenuates enhancement of glucocorticoid receptors in the prefrontal cortex in an animal model of post-traumatic stress disorder. Biol Mood Anxiety Disord. 2013; 3:22–23.
Article9. Shea A, Walsh C, Macmillan H, Steiner M. Child maltreatment and HPA axis dysregulation: relationship to major depressive disorder and post traumatic stress disorder in females. Psychoneuroendocrinology. 2005; 30:162–178.
Article10. Pitman RK. Overview of biological themes in PTSD. Ann N Y Acad Sci. 1997; 821:1–9.
Article11. Yamamoto S, Morinobu S, Takei S, Fuchikami M, Matsuki A, Yamawaki S, Liberzon I. Single prolonged stress: toward an animal model of posttraumatic stress disorder. Depress Anxiety. 2009; 26:1110–1117.
Article12. Eskandarian S, Vafaei AA, Vaezi GH, Taherian F, Kashefi A, Rashidy-Pour A. Effects of systemic administration of oxytocin on contextual fear extinction in a rat model of post-traumatic stress disorder. Basic Clin Neurosci. 2013; 4:315–322.13. Kohda K, Harada K, Kato K, Hoshino A, Motohashi J, Yamaji T, Morinobu S, Matsuoka N, Kato N. Glucocorticoid receptor activation is involved in producing abnormal phenotypes of single-prolonged stress rats: a putative post-traumatic stress disorder model. Neuroscience. 2007; 148:22–33.
Article14. Li H, Yang X, Shi W, Ma Z, Feng GK, Yin YL, Fan YX, Jiang J. Protective effects of tetramethylpyrazine on cerebrovascular regulations in rats with chronic alcoholic encephalopathy. Biomed Environ Sci. 2015; 28:691–695.15. Cheng XR, Zhang L, Hu JJ, Sun L, Du GH. Neuroprotective effects of tetramethylpyrazine on hydrogen peroxide-induced apoptosis in PC12 cells. Cell Biol Int. 2007; 31:438–443.
Article16. Guan D, Su Y, Li Y, Wu C, Meng Y, Peng X, Cui Y. Tetramethylpyrazine inhibits CoCl2-induced neurotoxicity through enhancement of Nrf2/GCLc/GSH and suppression of HIF1a/NOX2/ROS pathways. J Neurochem. 2015; 134:551–565.17. Wu W, Yu X, Luo XP, Yang SH, Zheng D. Tetramethylpyrazine protects against scopolamine-induced memory impairments in rats by reversing the cAMP/PKA/CREB pathway. Behav Brain Res. 2013; 253:212–216.
Article18. Ding Y, Hou X, Chen L, Li H, Tang Y, Zhou H, Zhao S, Zheng Y. Protective action of tetramethylpyrazine on the medulla oblongata in rats with chronic hypoxia. Auton Neurosci. 2013; 173:45–52.
Article19. Xu D, Chen H, Mak S, Hu S, Tsim KWK, Hu Y, Sun Y, Zhang G, Wang Y, Zhang Z, Han Y. Neuroprotection against glutamate-induced excitotoxicity and induction of neurite outgrowth by T-00, a novel multifunctional derivative of tetramethylpyrazine in neuronal cell models. Neurochem Int. 2016; 99:194–205.20. Zhang H, Sun R, Liu XY, Shi XM, Wang WF, Yu LG, Guo XL. A tetramethylpyrazine piperazine derivate CXC137 prevents cell injury in SH-SY5Y cells and improves memory dysfunction of rats with vascular Dementia. Neurochem Res. 2014; 39:276–286.
Article21. Xiao X, Liu Y, Qi C, Qiu F, Chen X, Zhang J, Yang P. Neuroprotection and enhanced neurogenesis by tetramethylpyrazine in adult rat brain after focal ischemia. Neurol Res. 2010; 32:547–555.
Article22. Zhang C, Wang SZ, Zuo PP, Cui X, Cai J. Protective effect of tetramethylpyrazine on learning and memory function in D-galactose-lesioned mice. Chin Med Sci J. 2004; 19:180–184.23. Zhang T, Gu J, Wu L, Li N, Sun Y, Yu P, Wang Y, Zhang G, Zhang Z. Neuroprotective and axonal outgrowth-promoting effects of tetramethylpyrazine nitrone in chronic cerebral hypoperfusion rats and primary hippocampal neurons exposed to hypoxia. Neuropharmacology. 2017; 118:137–147.
Article24. Lu F, Li X, Li W, Wei K, Yao Y, Zhang Q, Liang X, Zhang J. Tetramethylpyrazine reverses intracerebroventricular streptozotocin-induced memory deficits by inhibiting GSK-3b. Acta Biochim Biophys Sin (Shanghai). 2017; 49:722–728.25. Li SY, Jia YH, Sun WG, Tang Y, An GS, Ni JH, Jia HT. Stabilization of mitochondrial function by tetramethylpyrazine protects against kainate-induced oxidative lesions in the rat hippocampus. Free Radic Biol Med. 2010; 48:597–608.
Article26. Liu HT, Du YG, He JL, Chen WJ, Li WM, Yang Z, Wang YX, Yu C. Tetramethylpyrazine inhibits production of nitric oxide and inducible nitric oxide synthase in lipopolysaccharide-induced N9 microglial cells through blockade of MAPK and PI3K/Akt signaling pathways, and suppression of intracellular reactive oxygen species. J Ethnopharmacol. 2010; 129:335–343.
Article27. Tan Z. Neural protection by naturopathic compounds-an example of tetramethylpyrazine from retina to brain. J Ocul Biol Dis Infor. 2009; 2:57–64.
Article28. Fan LH, Wang KZ, Cheng B, Wang CS, Dang XQ. Anti-apoptotic and neuroprotective effects of Tetramethylpyrazine following spinal cord ischemia in rabbits. BMC Neurosci. 2006; 7:48–56.
Article29. Shih YH, Wu SL, Chiou WF, Ku HH, Ko TL, Fu YS. Protective effects of tetramethylpyrazine on kainate-induced excitotoxicity in hippocampal culture. Neuroreport. 2002; 13:515–519.
Article30. Lee B, Sur B, Yeom M, Shim I, Lee H, Hahm DH. L-tetrahydropalmatine ameliorates development of anxiety and depression-related symptoms induced by single prolonged stress in rats. Biomol Ther (Seoul). 2014; 22:213–222.31. Lee B, Sur B, Yeom M, Shim I, Lee H, Hahm DH. Effects of systemic administration of ibuprofen on stress response in a rat model of post-traumatic stress disorder. Korean J Physiol Pharmacol. 2016; 20:357–366.
Article32. Davies DR, Olson D, Meyer DL, Scholl JL, Watt MJ, Manzerra P, Renner KJ, Forster GL. Mild traumatic brain injury with social defeat stress alters anxiety, contextual fear extinction, and limbic monoamines in adult rats. Front Behav Neurosci. 2016; 10:71–80.
Article33. Eagle AL, Fitzpatrick CJ, Perrine SA. Single prolonged stress impairs social and object novelty recognition in rats. Behav Brain Res. 2013; 256:591–597.
Article34. Peng Y, Feng SF, Wang Q, Wang HN, Hou WG, Xiong L, Luo ZJ, Tan QR. Hyperbaric oxygen preconditioning ameliorates anxiety-like behavior and cognitive impairments via upregulation of thioredoxin reductases in stressed rats. Prog Neuropsychopharmacol Biol Psychiatry. 2010; 34:1018–1025.
Article35. Knox D, George SA, Fitzpatrick CJ, Rabinak CA, Maren S, Liberzon I. Single prolonged stress disrupts retention of extinguished fear in rats. Learn Mem. 2012; 19:43–49.
Article36. Yamamoto S, Morinobu S, Fuchikami M, Kurata A, Kozuru T, Yamawaki S. Effects of single prolonged stress and D-cycloserine on contextual fear extinction and hippocampal NMDA receptor expression in a rat model of PTSD. Neuropsychopharmacology. 2008; 33:2108–2116.
Article37. Khan S, Liberzon I. Topiramate attenuates exaggerated acoustic startle in an animal model of PTSD. Psychopharmacology (Berl). 2004; 172:225–229.
Article38. Hohlbaum K, Bert B, Dietze S, Palme R, Fink H, Thöne-Reineke C. Severity classification of repeated isoflurane anesthesia in C57BL/6JRj mice-Assessing the degree of distress. PLoS One. 2017; 12:e0179588.
Article39. Rau V, Oh I, Laster M, Eger EI 2nd, Fanselow MS. Isoflurane suppresses stress-enhanced fear learning in a rodent model of post-traumatic stress disorder. Anesthesiology. 2009; 110:487–495.
Article40. Shafia S, Vafaei AA, Samaei SA, Bandegi AR, Rafiei A, Valadan R, Hosseini-Khah Z, Mohammadkhani R, Rashidy-Pour A. Effects of moderate treadmill exercise and fluoxetine on behavioural and cognitive deficits, hypothalamic-pituitary-adrenal axis dysfunction and alternations in hippocampal BDNF and mRNA expression of apoptosis - related proteins in a rat model of post-traumatic stress disorder. Neurobiol Learn Mem. 2017; 139:165–178.
Article41. Serova LI, Laukova M, Alaluf LG, Sabban EL. Intranasal infusion of melanocortin receptor four (MC4R) antagonist to rats ameliorates development of depression and anxiety related symptoms induced by single prolonged stress. Behav Brain Res. 2013; 250:139–147.
Article42. Lever C, Burton S, O'Keefe J. Rearing on hind legs, environmental novelty, and the hippocampal formation. Rev Neurosci. 2006; 17:111–133.
Article43. Crusio WE. Genetic dissection of mouse exploratory behaviour. Behav Brain Res. 2001; 125:127–132.
Article44. Sturman O, Germain PL, Bohacek J. Exploratory rearing: a context- and stress-sensitive behavior recorded in the open-field test. Stress. 2018; 1–10.
Article45. Torres-Lista V, Parrado-Fernández C, Alvarez-Montón I, Frontiñán-Rubio J, Durán-Prado M, Peinado JR, Johansson B, Alcaín FJ, Giménez-Llort L. Neophobia, NQO1 and SIRT1 as premorbid and prodromal indicators of AD in 3xTg-AD mice. Behav Brain Res. 2014; 271:140–146.
Article46. Choi YJ, Kim JY, Jin WP, Kim YT, Lee JH, Jahng JW. Anxiolytic efficacy of repeated oral capsaicin in rats with partial aberration of oral sensory relay to brain. Arch Oral Biol. 2015; 60:989–997.
Article47. Chen LJ, Shen BQ, Liu DD, Li ST. The effects of early-life predator stress on anxiety- and depression-like behaviors of adult rats. Neural Plast. 2014; DOI: 10.1155/2014/163908.
Article48. Kabuki Y, Mizobe Y, Yamada S, Furuse M. Dietary l-tyrosine alleviates the behavioral alterations induced by social isolation stress in mice. Brain Res Bull. 2009; 80:389–396.
Article49. Kozlovsky N, Matar MA, Kaplan Z, Zohar J, Cohen H. The role of the galaninergic system in modulating stress-related responses in an animal model of posttraumatic stress disorder. Biol Psychiatry. 2009; 65:383–391.
Article50. Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005; 4:775–790.
Article51. Zhang XG, Zhang H, Liang XL, Liu Q, Wang HY, Cao B, Cao J, Liu S, Long YJ, Xie WY, Peng DZ. Epigenetic mechanism of maternal post-traumatic stress disorder in delayed rat offspring development: dysregulation of methylation and gene expression. Genet Mol Res. 2016; 15:1–10.
Article52. Wang HT, Han F, Shi YX. Activity of the 5-HT1A receptor is involved in the alteration of glucocorticoid receptor in hippocampus and corticotropin-releasing factor in hypothalamus in SPS rats. Int J Mol Med. 2009; 24:227–231.
Article53. Aykaç A, Aydın B, Cabadak H, Gören MZ. The change in muscarinic receptor subtypes in different brain regions of rats treated with fluoxetine or propranolol in a model of post-traumatic stress disorder. Behav Brain Res. 2012; 232:124–129.
Article54. Nikolaus S, Antke C, Beu M, Müller HW. Cortical GABA, striatal dopamine and midbrain serotonin as the key players in compulsive and anxiety disorders--results from in vivo imaging studies. Rev Neurosci. 2010; 21:119–139.
Article55. Castro JE, Diessler S, Varea E, Márquez C, Larsen MH, Cordero MI, Sandi C. Personality traits in rats predict vulnerability and resilience to developing stress-induced depression-like behaviors, HPA axis hyper-reactivity and brain changes in pERK1/2 activity. Psychoneuroendocrinology. 2012; 37:1209–1223.
Article56. Meyer EM, Long V, Fanselow MS, Spigelman I. Stress increases voluntary alcohol intake, but does not alter established drinking habits in a rat model of posttraumatic stress disorder. Alcohol Clin Exp Res. 2013; 37:566–574.
Article57. Roth MK, Bingham B, Shah A, Joshi A, Frazer A, Strong R, Morilak DA. Effects of chronic plus acute prolonged stress on measures of coping style, anxiety, and evoked HPA-axis reactivity. Neuropharmacology. 2012; 63:1118–1126.
Article58. Leng YF, Gao XM, Wang SX, Xing YH. Effects of tetramethylpyrazine on neuronal apoptosis in the superficial dorsal horn in a rat model of neuropathic pain. Am J Chin Med. 2012; 40:1229–1239.
Article59. Gao B, Lin X, Jing H, Fan J, Ji C, Jie Q, Zheng C, Wang D, Xu X, Hu Y, Lu W, Luo Z, Yang L. Local delivery of tetramethylpyrazine eliminates the senescent phenotype of bone marrow mesenchymal stromal cells and creates an anti-inflammatory and angiogenic environment in aging mice. Aging Cell. 2018; 17:e12741.
Article60. Yang QH, Liang Y, Xu Q, Zhang Y, Xiao L, Si LY. Protective effect of tetramethylpyrazine isolated from Ligusticum chuanxiong on nephropathy in rats with streptozotocin-induced diabetes. Phytomedicine. 2011; 18:1148–1152.
Article61. Kim M, Kim SO, Lee M, Lee JH, Jung WS, Moon SK, Kim YS, Cho KH, Ko CN, Lee EH. Tetramethylpyrazine, a natural alkaloid, attenuates pro-inflammatory mediators induced by amyloid b and interferon-g in rat brain microglia. Eur J Pharmacol. 2014; 740:504–511.62. Zhang Y, Ren P, Kang Q, Liu W, Li S, Li P, Liu H, Shang J, Zhang L, Gong Y, Zhou M. Effect of tetramethylpyrazine on atherosclerosis and SCAP/SREBP-1c signaling pathway in ApoE-/- mice fed with a high-fat diet. Evid Based Complement Alternat Med. 2017; DOI: 10.1155/2017/3121989.63. Zhao X, Jiang J, Yang G, Huang J, Yang G, He G, Chu Z, Hang T, Fan G. Profiling and preparation of metabolites from pyragrel in human urine by online solid-phase extraction coupled with high performance liquid chromatography tandem mass spectrometry followed by a macroporous resin-based purification approach. Molecules. 2017; 22:E494.
Article64. Wang GF, Shi CG, Sun MZ, Wang L, Wu SX, Wang HF, Xu ZQ, Chen DM. Tetramethylpyrazine attenuates atherosclerosis development and protects endothelial cells from ox-LDL. Cardiovasc Drugs Ther. 2013; 27:199–210.
Article65. Chen L, Cheng L, Wei X, Yuan Z, Wu Y, Wang S, Ren Z, Liu X, Liu H. Tetramethylpyrazine analogue CXC195 protects against dopaminergic neuronal apoptosis via activation of PI3K/Akt/GSK3β signaling pathway in 6-OHDA-induced Parkinson's disease mice. Neurochem Res. 2017; 42:1141–1150.
Article66. Jiang Y, Liu C, Chen W, Wang H, Wang C, Lin N. Tetramethylpyrazine enhances vascularization and prevents osteonecrosis in steroid-treated rats. Biomed Res Int. 2015; DOI: 10.1155/2015/315850.
Article67. Moon SK, Kim YS, Park SU, Jung WS, Ko CN, Cho KH, Bae HS. Effect of gastrodia elata BL water extract on human cerebral blood flow using transcranial doppler. Korean Oriental Medicine. 2005; 26:115–122.68. Guo B, Xu D, Duan H, Du J, Zhang Z, Lee SM, Wang Y. Therapeutic effects of multifunctional tetramethylpyrazine nitrone on models of Parkinson’s disease in vitro and in vivo. Biol Pharm Bull. 2014; 37:274–285.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Post-Traumatic Stress Disorder, Depression and Anxiety among North Korean Refugees: A Meta-Analysis
- Prevalence and Risk Factors of Anxiety, Depression, and Post-Traumatic Stress Disorder in Critical Care Survivors
- Effects of systemic administration of ibuprofen on stress response in a rat model of post-traumatic stress disorder
- L-Tetrahydropalmatine Ameliorates Development of Anxiety and Depression-Related Symptoms Induced by Single Prolonged Stress in Rats
- Post-Traumatic stress disorder