Korean J Physiol Pharmacol.
2018 Sep;22(5):503-511. 10.4196/kjpp.2018.22.5.503.
Lysophosphatidic acid enhances breast cancer cells-mediated osteoclastogenesis
- Affiliations
-
- 1Institute for Skeletal Aging & Orthopedic Surgery, Hallym University-Chuncheon Sacred Heart Hospital, Chuncheon 24252, Korea. jsnam88@hallym.ac.kr 123sslee@gmail.com
- KMID: 2419000
- DOI: http://doi.org/10.4196/kjpp.2018.22.5.503
Abstract
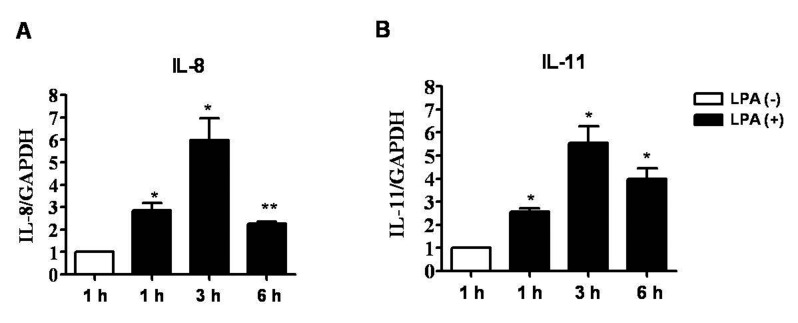
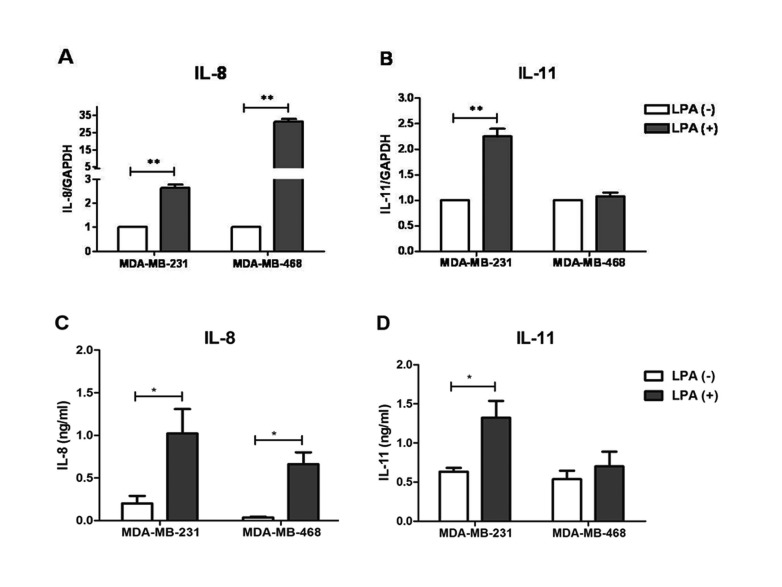
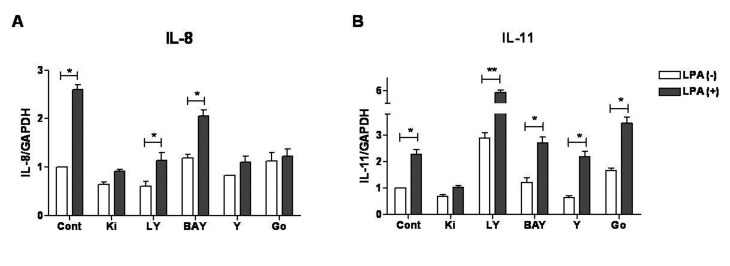
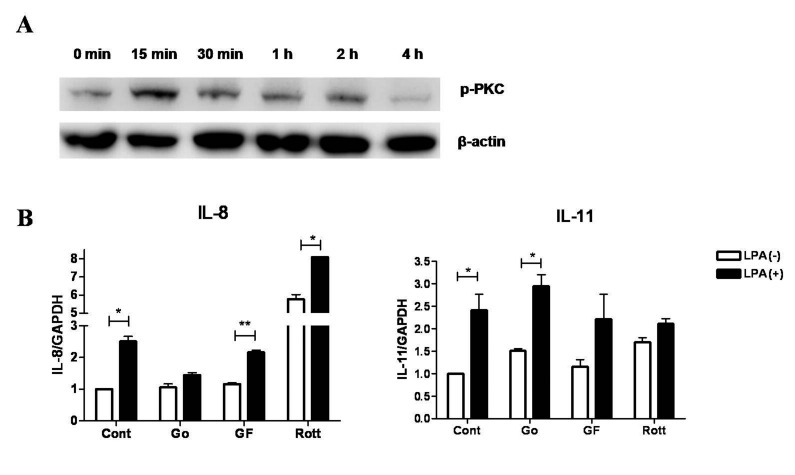
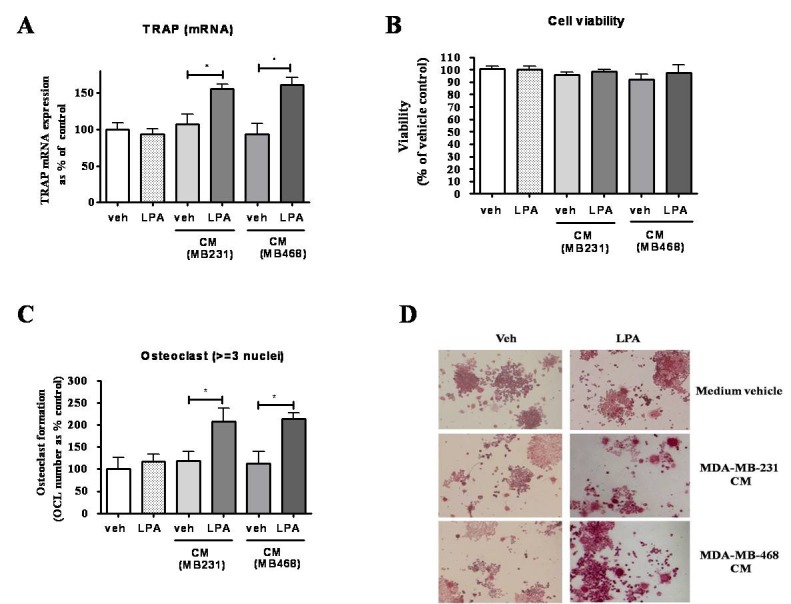
- Lysophosphatidic acid (LPA) is known to play a critical role in breast cancer metastasis to bone. In this study, we tried to investigate any role of LPA in the regulation of osteoclastogenic cytokines from breast cancer cells and the possibility of these secretory factors in affecting osteoclastogenesis. Effect of secreted cytokines on osteoclastogenesis was analyzed by treating conditioned media from LPA-stimulated breast cancer cells to differentiating osteoclasts. Result demonstrated that IL-8 and IL-11 expression were upregulated in LPA-treated MDA-MB-231 cells. IL-8 was induced in both MDA-MB-231 and MDA-MB-468, however, IL-11 was induced only in MDA-MB-231, suggesting differential LPARs participation in the expression of these cytokines. Expression of IL-8 but not IL-11 was suppressed by inhibitors of PI3K, NFkB, ROCK and PKC pathways. In the case of PKC activation, it was observed that PKCδ and PKCμ might regulate LPA-induced expression of IL-11 and IL-8, respectively, by using specific PKC subtype inhibitors. Finally, conditioned Medium from LPA-stimulated breast cancer cells induced osteoclastogenesis. In conclusion, LPA induced the expression of osteolytic cytokines (IL-8 and IL-11) in breast cancer cells by involving different LPA receptors. Enhanced expression of IL-8 by LPA may be via ROCK, PKCu, PI3K, and NFkB signaling pathways, while enhanced expression of IL-11 might involve PKCδ signaling pathway. LPA has the ability to enhance breast cancer cells-mediated osteoclastogenesis by inducing the secretion of cytokines such as IL-8 and IL-11.
MeSH Terms
Figure
Reference
-
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108. PMID: 25651787.
Article2. Lin Y, Huang R, Chen L, Li S, Shi Q, Jordan C, Huang RP. Identification of interleukin-8 as estrogen receptor-regulated factor involved in breast cancer invasion and angiogenesis by protein arrays. Int J Cancer. 2004; 109:507–515. PMID: 14991571.
Article3. Yao C, Lin Y, Chua MS, Ye CS, Bi J, Li W, Zhu YF, Wang SM. Interleukin-8 modulates growth and invasiveness of estrogen receptornegative breast cancer cells. Int J Cancer. 2007; 121:1949–1957. PMID: 17621625.
Article4. Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003; 170:3369–3376. PMID: 12626597.
Article5. Martin D, Galisteo R, Gutkind JS. CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) complex. J Biol Chem. 2009; 284:6038–6042. PMID: 19112107.6. Marusyk A, Tabassum DP, Altrock PM, Almendro V, Michor F, Polyak K. Non-cell-autonomous driving of tumour growth supports sub-clonal heterogeneity. Nature. 2014; 514:54–58. PMID: 25079331.
Article7. Benoy IH, Salgado R, Van Dam P, Geboers K, Van Marck E, Scharpé S, Vermeulen PB, Dirix LY. Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin Cancer Res. 2004; 10:7157–7162. PMID: 15534087.
Article8. Sotiriou C, Lacroix M, Lespagnard L, Larsimont D, Paesmans M, Body JJ. Interleukins-6 and -11 expression in primary breast cancer and subsequent development of bone metastases. Cancer Lett. 2001; 169:87–95. PMID: 11410329.
Article9. Morgan H, Tumber A, Hill PA. Breast cancer cells induce osteoclast formation by stimulating host IL-11 production and downregulating granulocyte/macrophage colony-stimulating factor. Int J Cancer. 2004; 109:653–660. PMID: 14999770.
Article10. McCoy EM, Hong H, Pruitt HC, Feng X. IL-11 produced by breast cancer cells augments osteoclastogenesis by sustaining the pool of osteoclast progenitor cells. BMC Cancer. 2013; 13:16. PMID: 23311882.
Article11. Bendre MS, Montague DC, Peery T, Akel NS, Gaddy D, Suva LJ. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone. 2003; 33:28–37. PMID: 12919697.
Article12. Boucharaba A, Serre CM, Guglielmi J, Bordet JC, Clézardin P, Peyruchaud O. The type 1 lysophosphatidic acid receptor is a target for therapy in bone metastases. Proc Natl Acad Sci U S A. 2006; 103:9643–9648. PMID: 16769891.
Article13. Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003; 3:582–591. PMID: 12894246.
Article14. Chen M, Towers LN, O'Connor KL. LPA2 (EDG4) mediates Rho-dependent chemotaxis with lower efficacy than LPA1 (EDG2) in breast carcinoma cells. Am J Physiol Cell Physiol. 2007; 292:C1927–C1933. PMID: 17496233.
Article15. Liu S, Umezu-Goto M, Murph M, Lu Y, Liu W, Zhang F, Yu S, Stephens LC, Cui X, Murrow G, Coombes K, Muller W, Hung MC, Perou CM, Lee AV, Fang X, Mills GB. Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell. 2009; 15:539–550. PMID: 19477432.
Article16. Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005; 434:843–850. PMID: 15829953.
Article17. Li Y, Welm B, Podsypanina K, Huang S, Chamorro M, Zhang X, Rowlands T, Egeblad M, Cowin P, Werb Z, Tan LK, Rosen JM, Varmus HE. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci U S A. 2003; 100:15853–15858. PMID: 14668450.
Article18. Liu S, Murph M, Panupinthu N, Mills GB. ATX-LPA receptor axis in inflammation and cancer. Cell Cycle. 2009; 8:3695–3701. PMID: 19855166.
Article19. Panupinthu N, Lee HY, Mills GB. Lysophosphatidic acid production and action: critical new players in breast cancer initiation and progression. Br J Cancer. 2010; 102:941–946. PMID: 20234370.
Article20. Boucharaba A, Serre CM, Grès S, Saulnier-Blache JS, Bordet JC, Guglielmi J, Clézardin P, Peyruchaud O. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J Clin Invest. 2004; 114:1714–1725. PMID: 15599396.
Article21. Du J, Sun C, Hu Z, Yang Y, Zhu Y, Zheng D, Gu L, Lu X. Lysophosphatidic acid induces MDA-MB-231 breast cancer cells migration through activation of PI3K/PAK1/ERK signaling. PLoS One. 2010; 5:e15940. PMID: 21209852.
Article22. Samadi N, Bekele RT, Goping IS, Schang LM, Brindley DN. Lysophosphatidate induces chemo-resistance by releasing breast cancer cells from taxol-induced mitotic arrest. PLoS One. 2011; 6:e20608. PMID: 21647386.
Article23. Hama K, Aoki J, Fukaya M, Kishi Y, Sakai T, Suzuki R, Ohta H, Yamori T, Watanabe M, Chun J, Arai H. Lysophosphatidic acid and autotaxin stimulate cell motility of neoplastic and non-neoplastic cells through LPA1. J Biol Chem. 2004; 279:17634–17639. PMID: 14744855.
Article24. Swamydas M, Nguyen D, Allen LD, Eddy J, Dréau D. Progranulin stimulated by LPA promotes the migration of aggressive breast cancer cells. Cell Commun Adhes. 2011; 18:119–130. PMID: 22176685.
Article25. Brünner N, Boysen B, Rømer J, Spang-Thomsen M. The nude mouse as an in vivo model for human breast cancer invasion and metastasis. Breast Cancer Res Treat. 1993; 24:257–264. PMID: 8435480.26. Chekhun S, Bezdenezhnykh N, Shvets J, Lukianova N. Expression of biomarkers related to cell adhesion, metastasis and invasion of breast cancer cell lines of different molecular subtype. Exp Oncol. 2013; 35:174–179. PMID: 24084454.27. Dickson RB, Bates SE, McManaway ME, Lippman ME. Characterization of estrogen responsive transforming activity in human breast cancer cell lines. Cancer Res. 1986; 46:1707–1713. PMID: 2418952.28. Hwang YS, Lee SK, Park KK, Chung WY. Secretion of IL-6 and IL-8 from lysophosphatidic acid-stimulated oral squamous cell carcinoma promotes osteoclastogenesis and bone resorption. Oral Oncol. 2012; 48:40–48. PMID: 21925926.
Article29. Shimada H, Rajagopalan LE. Rho-kinase mediates lysophosphatidic acid-induced IL-8 and MCP-1 production via p38 and JNK pathways in human endothelial cells. FEBS Lett. 2010; 584:2827–2832. PMID: 20434448.
Article30. Hartman ZC, Poage GM, den Hollander P, Tsimelzon A, Hill J, Panupinthu N, Zhang Y, Mazumdar A, Hilsenbeck SG, Mills GB, Brown PH. Growth of triple-negative breast cancer cells relies upon coordinate autocrine expression of the proinflammatory cytokines IL-6 and IL-8. Cancer Res. 2013; 73:3470–3480. PMID: 23633491.
Article31. Chen RJ, Chen SU, Chou CH, Lin MC. Lysophosphatidic acid receptor 2/3-mediated IL-8-dependent angiogenesis in cervical cancer cells. Int J Cancer. 2012; 131:789–802. PMID: 21964883.
Article32. Gupta J, Robbins J, Jilling T, Seth P. TGFβ-dependent induction of interleukin-11 and interleukin-8 involves SMAD and p38 MAPK pathways in breast tumor models with varied bone metastases potential. Cancer Biol Ther. 2011; 11:311–316. PMID: 21099351.
Article33. Matsumoto T, Kuriwaka-Kido R, Kondo T, Endo I, Kido S. Regulation of osteoblast differentiation by interleukin-11 via AP-1 and Smad signaling. Endocr J. 2012; 59:91–101. PMID: 21931225.34. Kido S, Kuriwaka-Kido R, Umino-Miyatani Y, Endo I, Inoue D, Taniguchi H, Inoue Y, Imamura T, Matsumoto T. Mechanical stress activates Smad pathway through PKCδ to enhance interleukin-11 gene transcription in osteoblasts. PLoS One. 2010; 5:e13090. PMID: 20927330.
Article35. Brenner W, Beitz S, Schneider E, Benzing F, Unger RE, Roos FC, Thüroff JW, Hampel C. Adhesion of renal carcinoma cells to endothelial cells depends on PKCmu. BMC Cancer. 2010; 10:183. PMID: 20459627.
Article36. David M, Wannecq E, Descotes F, Jansen S, Deux B, Ribeiro J, Serre CM, Grès S, Bendriss-Vermare N, Bollen M, Saez S, Aoki J, Saulnier-Blache JS, Clézardin P, Peyruchaud O. Cancer cell expression of autotaxin controls bone metastasis formation in mouse through lysophosphatidic acid-dependent activation of osteoclasts. PLoS One. 2010; 5:e9741. PMID: 20305819.
Article37. Kang Y, He W, Tulley S, Gupta GP, Serganova I, Chen CR, Manova-Todorova K, Blasberg R, Gerald WL, Massagué J. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc Natl Acad Sci U S A. 2005; 102:13909–13914. PMID: 16172383.
Article38. Mu H, Calderone TL, Davies MA, Prieto VG, Wang H, Mills GB, Bar-Eli M, Gershenwald JE. Lysophosphatidic acid induces lymphangiogenesis and IL-8 production in vitro in human lymphatic endothelial cells. Am J Pathol. 2012; 180:2170–2181. PMID: 22465753.
Article39. Yung YC, Stoddard NC, Chun J. LPA receptor signaling: pharmacology, physiology, and pathophysiology. J Lipid Res. 2014; 55:1192–1214. PMID: 24643338.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Action and Signaling of Lysophosphatidylethanolamine in MDA-MB-231 Breast Cancer Cells
- Promising Pharmacological Directions in the World of Lysophosphatidic Acid Signaling
- Expression of Osteoprotegerin and RANK Ligand in Breast Cancer Bone Metastasis
- The Inhibitory Effects of Forsythia Koreana Extracts on the Metastatic Ability of Breast Cancer Cells and Bone Resorption by Osteoclasts
- Facile Titrimetric Assay of Lysophosphatidic Acid in Human Serum and Plasma for Ovarian Cancer Detection