Yonsei Med J.
2018 Jan;59(1):51-56. 10.3349/ymj.2018.59.1.51.
14-3-3ζ Overexpression is Associated with Poor Prognosis in Ovarian Cancer
- Affiliations
-
- 1Department of Obstetrics and Gynecology, School of Medicine, Ewha Womans University, Seoul, Korea. goodmorning@ewha.ac.kr
- 2Innovative Research Center for Control and Prevention of Women's Cancer, Ewha Womans University Mokdong Hospital, Seoul, Korea.
- 3Department of Pathology, School of Medicine, Ewha Womans University, Seoul, Korea.
- KMID: 2418848
- DOI: http://doi.org/10.3349/ymj.2018.59.1.51
Abstract
- PURPOSE
14-3-3ζ regulates cell signaling, cell cycle progression, and apoptosis, and its overexpression is associated with disease recurrence and poor clinical outcomes in some solid tumors. However, its clinicopathological role in ovarian cancer is unknown. Our goal was to investigate whether 14-3-3ζ is associated with ovarian cancer prognosis.
MATERIALS AND METHODS
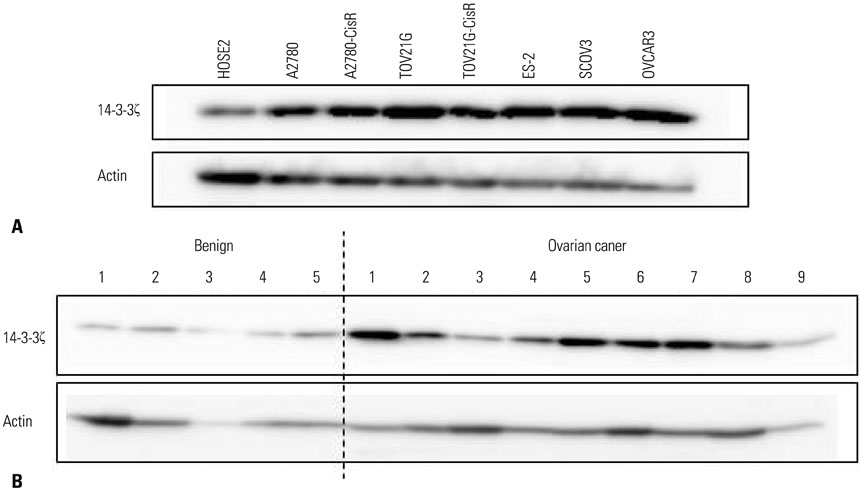
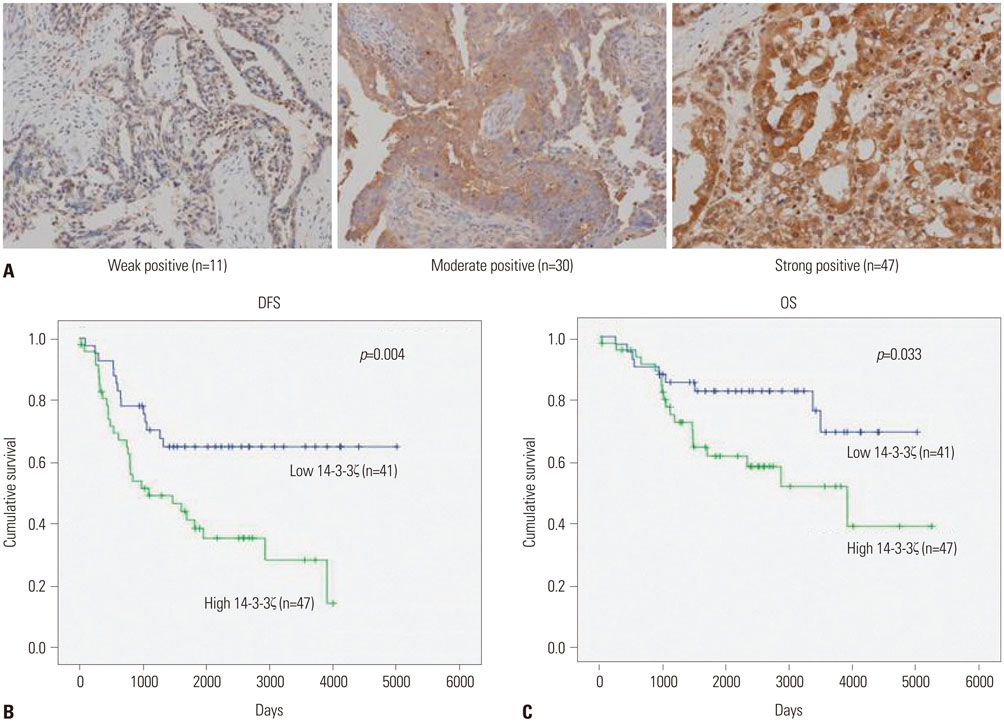
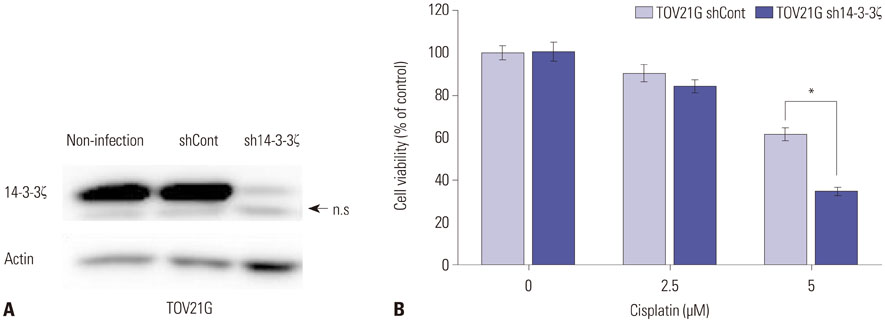
We examined 14-3-3ζ expression by immunohistochemistry in ovarian cancer tissues obtained from 88 ovarian cancer patients. The examined tissues were of various histologies and stages. 14-3-3ζ expression was also analyzed by western blot in seven ovarian cancer cell lines and a primary ovary epithelial cell line. Cell viability was measured using an MTS-based assay following cisplatin treatment.
RESULTS
Among the ovarian cancer samples, 53.4% (47/88) showed high 14-3-3ζ expression, and 14-3-3ζ overexpression was positively correlated with more advanced pathologic stages and grades. 14-3-3ζ overexpression was also significantly associated with poor disease-free survival (DFS) and overall survival (OS) of ovarian cancer patients. Median DFS and OS were 1088 and 3905 days, respectively, in the high 14-3-3ζ expression group, but not reached in the low 14-3-3ζ expression group (p=0.004 and p=0.033, log-rank test, respectively). Downregulating 14-3-3ζ by RNA interference in ovarian cancer cells led to enhanced sensitivity to cisplatin-induced cell death.
CONCLUSION
14-3-3ζ overexpression might be a potential prognostic biomarker for ovarian cancer, and the inhibition of 14-3-3ζ could be a therapeutic option that enhances the antitumor activity of cisplatin.
Keyword
MeSH Terms
Figure
Reference
-
1. Partridge E, Kreimer AR, Greenlee RT, Williams C, Xu JL, Church TR, et al. Results from four rounds of ovarian cancer screening in a randomized trial. Obstet Gynecol. 2009; 113:775–782.
Article2. Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006; 354:34–43.
Article3. Markman M, Rothman R, Hakes T, Reichman B, Hoskins W, Rubin S, et al. Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol. 1991; 9:389–393.
Article4. Aitken A, Jones D, Soneji Y, Howell S. 14-3-3 proteins: biological function and domain structure. Biochem Soc Trans. 1995; 23:605–611.
Article5. Morrison DK. The 14-3-3 proteins: integrators of diverse signaling cues that impact cell fate and cancer development. Trends Cell Biol. 2009; 19:16–23.
Article6. Tzivion G, Avruch J. 14-3-3 proteins: active cofactors in cellular regulation by serine/threonine phosphorylation. J Biol Chem. 2002; 277:3061–3064.
Article7. Yaffe MB. How do 14-3-3 proteins work?–Gatekeeper phosphorylation and the molecular anvil hypothesis. FEBS Lett. 2002; 513:53–57.
Article8. van Hemert MJ, Steensma HY, van Heusden GP. 14-3-3 proteins: key regulators of cell division, signalling and apoptosis. Bioessays. 2001; 23:936–946.
Article9. Tzivion G, Gupta VS, Kaplun L, Balan V. 14-3-3 proteins as potential oncogenes. Semin Cancer Biol. 2006; 16:203–213.
Article10. Matta A, DeSouza LV, Ralhan R, Siu KW. Small interfering RNA targeting 14-3-3ζ increases efficacy of chemotherapeutic agents in head and neck cancer cells. Mol Cancer Ther. 2010; 9:2676–2688.
Article11. Fan T, Li R, Todd NW, Qiu Q, Fang HB, Wang H, et al. Up-regulation of 14-3-3zeta in lung cancer and its implication as prognostic and therapeutic target. Cancer Res. 2007; 67:7901–7906.
Article12. Neal CL, Yao J, Yang W, Zhou X, Nguyen NT, Lu J, et al. 14-3-3zeta overexpression defines high risk for breast cancer recurrence and promotes cancer cell survival. Cancer Res. 2009; 69:3425–3432.
Article13. Watanabe N, Komatsu S, Ichikawa D, Miyamae M, Ohashi T, Okajima W, et al. Overexpression of YWHAZ as an independent prognostic factor in adenocarcinoma of the esophago-gastric junction. Am J Cancer Res. 2016; 6:2729–2736.14. Sasaki R, Narisawa-Saito M, Yugawa T, Fujita M, Tashiro H, Katabuchi H, et al. Oncogenic transformation of human ovarian surface epithelial cells with defined cellular oncogenes. Carcinogenesis. 2009; 30:423–431.
Article15. Lim MC, Moon EK, Shin A, Jung KW, Won YJ, Seo SS, et al. Incidence of cervical, endometrial, and ovarian cancer in Korea, 1999-2010. J Gynecol Oncol. 2013; 24:298–302.
Article16. Matta A, DeSouza LV, Shukla NK, Gupta SD, Ralhan R, Siu KW. Prognostic significance of head-and-neck cancer biomarkers previously discovered and identified using iTRAQ-labeling and multidimensional liquid chromatography-tandem mass spectrometry. J Proteome Res. 2008; 7:2078–2087.
Article17. Li Z, Zhao J, Du Y, Park HR, Sun SY, Bernal-Mizrachi L, et al. Down-regulation of 14-3-3zeta suppresses anchorage-independent growth of lung cancer cells through anoikis activation. Proc Natl Acad Sci U S A. 2008; 105:162–167.
Article18. Ralhan R, Masui O, Desouza LV, Matta A, Macha M, Siu KW. Identification of proteins secreted by head and neck cancer cell lines using LC-MS/MS: strategy for discovery of candidate serological biomarkers. Proteomics. 2011; 11:2363–2376.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Expression of HER-2/neu Oncoprotein in Endometrial Cancer
- Overexpression of hTERT and c-erbB-2 are correlated in ovarian epithelial cancer
- A Case of Recurrent Early-stage Epithelial Ovarian Cancer Presenting as Bone Metastasis
- Clinical Characteristics of Ovarian Metastasis from Colorectal Cancer
- Significance of p53 Overexpression in the Metastasis of Colorectal Cancer