Allergy Asthma Immunol Res.
2018 Sep;10(5):562-569. 10.4168/aair.2018.10.5.562.
Microarray-Based Multivariate Analysis of the Effectiveness of Sublingual Immunotherapy for Cedar Pollinosis
- Affiliations
-
- 1Department of Otorhinolaryngology, Nippon Medical School, Tokyo, Japan. m.gotoh@nms.ac.jp
- 2Allergy and Immunology Project, The Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan.
- 3Center for Life Science Research, University of Yamanashi, Yamanashi, Japan.
- KMID: 2418053
- DOI: http://doi.org/10.4168/aair.2018.10.5.562
Abstract
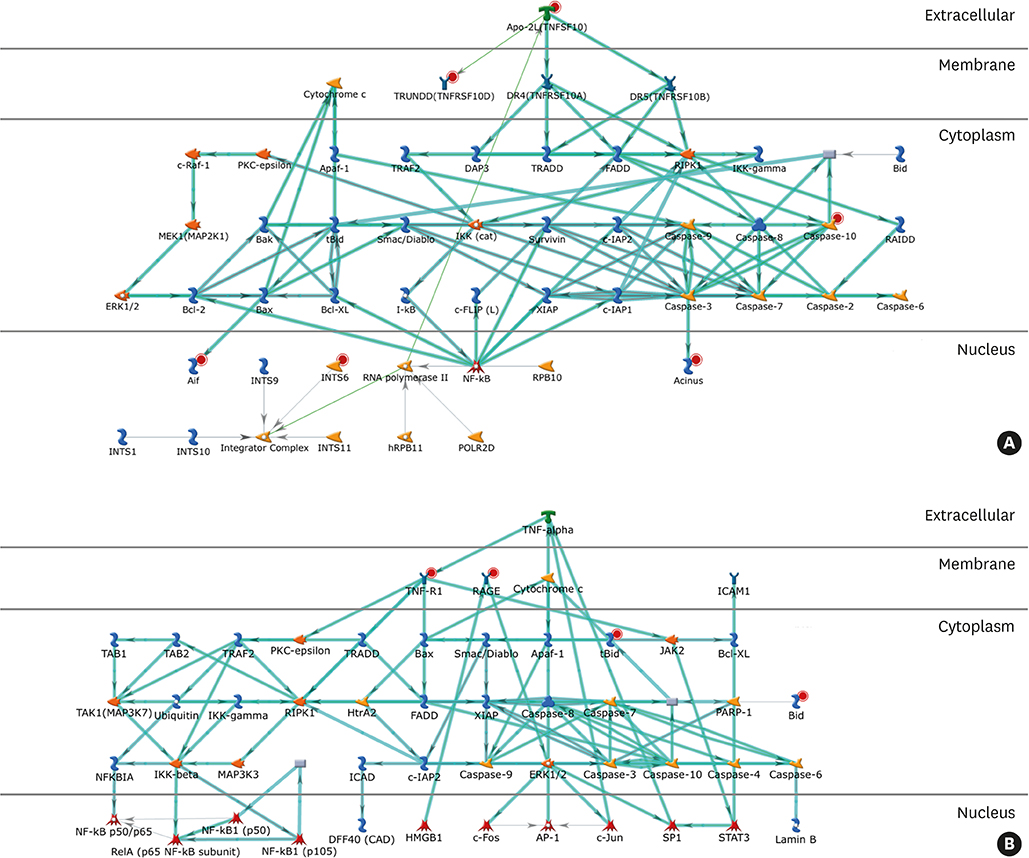
- Sublingual immunotherapy (SLIT) is an effective treatment for allergic diseases. However, the mechanism by which this therapy exhibits its efficacy has not been fully delineated. To elucidate the mechanisms of SLIT in the treatment of cedar pollinosis (CP), we performed a multivariate analysis of microarray data on mRNA expression in CD4⺠T cells and basophils. Although 2-year treatment with SLIT using cedar extracts was effective in >70% of patients with CP, the remaining patients did not respond to this therapy. The mRNA expression levels in peripheral CD4⺠T cells and basophils from both high- and non-responder patients before and after undergoing SLIT were comparatively studied using microarray analysis. By processing the data using serial multivariate analysis, an apoptosis pathway was extracted in both CD4⺠T cells and basophils. Conclusively, the strong treatment effectiveness of SLIT in patients with CP may be caused by the induction of apoptosis in CD4⺠T cells and basophils in these patients (Trial registry at University Hospital Medical Information Network Clinical Trials Registry Database, UMIN000016532).
Keyword
MeSH Terms
Figure
Reference
-
1. Fujieda S, Kurono Y, Okubo K, Ichimura K, Enomoto T, Kawauchi H, et al. Examination, diagnosis and classification for Japanese allergic rhinitis: Japanese guideline. Auris Nasus Larynx. 2012; 39:553–556.
Article2. Yamada T, Saito H, Fujieda S. Present state of Japanese cedar pollinosis: The national affliction. J Allergy Clin Immunol. 2014; 133:632–639.e5.
Article3. Sohn MH. Efficacy and safety of subcutaneous allergen immunotherapy for allergic rhinitis. Allergy Asthma Immunol Res. 2018; 10:1–3.
Article4. Mitobe Y, Yokomoto Y, Ohashi-Doi K. Safety evaluation of standardized allergen extract of Japanese cedar pollen for sublingual immunotherapy. Regul Toxicol Pharmacol. 2015; 71:529–540.
Article5. Horiguchi S, Okamoto Y, Yonekura S, Okawa T, Yamamoto H, Kunii N, et al. A randomized controlled trial of sublingual immunotherapy for Japanese cedar pollinosis. Int Arch Allergy Immunol. 2008; 146:76–84.
Article6. Okubo K, Gotoh M, Fujieda S, Okano M, Yoshida H, Morikawa H, et al. A randomized double-blind comparative study of sublingual immunotherapy for cedar pollinosis. Allergol Int. 2008; 57:265–275.
Article7. Fujimura T, Yonekura S, Horiguchi S, Taniguchi Y, Saito A, Yasueda H, et al. Increase of regulatory T cells and the ratio of specific IgE to total IgE are candidates for response monitoring or prognostic biomarkers in 2-year sublingual immunotherapy (SLIT) for Japanese cedar pollinosis. Clin Immunol. 2011; 139:65–74.
Article8. Kim ST. Outcome of sublingual immunotherapy in patients with allergic rhinitis sensitive to house dust mites. Allergy Asthma Immunol Res. 2015; 7:99–100.
Article9. Okamoto Y, Okubo K, Yonekura S, Hashiguchi K, Goto M, Otsuka T, et al. Efficacy and safety of sublingual immunotherapy for two seasons in patients with Japanese cedar pollinosis. Int Arch Allergy Immunol. 2015; 166:177–188.
Article10. Gotoh M, Kaminuma O, Nakaya A, Katayama K, Motoi Y, Watanabe N, et al. Identification of biomarker sets for predicting the efficacy of sublingual immunotherapy against pollen-induced allergic rhinitis. Int Immunol. 2017; 29:291–300.
Article11. Gotoh M, Kaminuma O, Nakaya A, Katayama K, Watanabe N, Saeki M, et al. Involvement of taste receptors in the effectiveness of sublingual immunotherapy. Allergol Int. Forthcoming. 2018.
Article12. Okubo K, Kurono Y, Fujieda S, Ogino S, Uchio E, Odajima H, et al. Japanese guideline for allergic rhinitis. Allergol Int. 2011; 60:171–189.
Article13. Okuda M. Epidemiology of Japanese cedar pollinosis throughout Japan. Ann Allergy Asthma Immunol. 2003; 91:288–296.
Article14. Di Lorenzo G, Mansueto P, Pacor ML, Rizzo M, Castello F, Martinelli N, et al. Evaluation of serum s-IgE/total IgE ratio in predicting clinical response to allergen-specific immunotherapy. J Allergy Clin Immunol. 2009; 123:1103–1110. 1110.e1–1110.e4.
Article15. Manzotti G, Lombardi C. Allergen immunotherapy as a drug: the new deal of grass allergen tablets from clinical trials to current practice. Eur Ann Allergy Clin Immunol. 2013; 45:34–42.16. Moingeon P, Mascarell L. Induction of tolerance via the sublingual route: mechanisms and applications. Clin Dev Immunol. 2012; 2012:623474.
Article17. Cappella A, Durham SR. Allergen immunotherapy for allergic respiratory diseases. Hum Vaccin Immunother. 2012; 8:1499–1512.
Article18. Jutel M, Van de Veen W, Agache I, Azkur KA, Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy and novel ways for vaccine development. Allergol Int. 2013; 62:425–433.
Article19. Allam JP, Bieber T, Novak N. Dendritic cells as potential targets for mucosal immunotherapy. Curr Opin Allergy Clin Immunol. 2009; 9:554–557.
Article20. Jutel M, Kosowska A, Smolinska S. Allergen immunotherapy: past, present, and future. Allergy Asthma Immunol Res. 2016; 8:191–197.
Article21. Guerra F, Carracedo J, Madueño JA, Sanchez-Guijo P, Ramírez R. Allergens induce apoptosis in lymphocytes from atopic patients. Hum Immunol. 1999; 60:840–847.
Article22. Guerra F, Carracedo J, Solana-Lara R, Sánchez-Guijo P, Ramírez R. TH2 lymphocytes from atopic patients treated with immunotherapy undergo rapid apoptosis after culture with specific allergens. J Allergy Clin Immunol. 2001; 107:647–653.
Article23. Gardner LM, O'Hehir RE, Rolland JM. High dose allergen stimulation of T cells from house dust mite-allergic subjects induces expansion of IFN-gamma+ T Cells, apoptosis of CD4+IL-4+ T cells and T cell anergy. Int Arch Allergy Immunol. 2004; 133:1–13.24. Kinikli G, Ateş A, Turgay M, Aydoğan N, Tokgöz G. Serum-soluble Fas antigen level in patients with allergic rhinitis: its relation to specific immunotherapy. Allergy Asthma Proc. 2006; 27:145–147.25. Ciepiela O, Zawadzka-Krajewska A, Kotula I, Wasik M, Demkow U. Sublingual immunotherapy in asthma does not influence lymphocyte sensitivity to Fas stimulation. Eur J Med Res. 2010; 15:Suppl 2. 17–20.
Article26. Falcone FH, Zillikens D, Gibbs BF. The 21st century renaissance of the basophil? Current insights into its role in allergic responses and innate immunity. Exp Dermatol. 2006; 15:855–864.
Article27. Jahnsen FL, Farkas L, Lund-Johansen F, Brandtzaeg P. Involvement of plasmacytoid dendritic cells in human diseases. Hum Immunol. 2002; 63:1201–1205.
Article28. Matsumoto K, Maeda A, Bochner BS, Wakiguchi H, Saito H. Induction of apoptosis in human basophils by anti-Fas antibody treatment in vitro. Int Arch Allergy Immunol. 2008; 146:Suppl 1. 40–46.29. Förster A, Falcone FH, Gibbs BF, Preussner LM, Fiebig BS, Altunok H, et al. Anti-Fas/CD95 and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) differentially regulate apoptosis in normal and neoplastic human basophils. Leuk Lymphoma. 2013; 54:835–842.
Article30. Karasuyama H, Yamanishi Y. Basophils have emerged as a key player in immunity. Curr Opin Immunol. 2014; 31:1–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sublingual Immunotherapy for Japanese Cedar Pollinosis Attenuates Asthma Exacerbation
- A practical view of immunotherapy for food allergy
- Gum pigmentation: an unusual adverse effect of sublingual immunotherapy
- Cost-Effectiveness Analysis of Immunotherapy in Patient with Allergic Rhinitis
- Sublingual immunotherapy for allergic rhinitis