Intest Res.
2018 Apr;16(2):223-232. 10.5217/ir.2018.16.2.223.
Trough level of infliximab is useful for assessing mucosal healing in Crohn's disease: a prospective cohort study
- Affiliations
-
- 1Department of Gastroenterology, Fukuoka University Chikushi Hospital, Chikushino, Japan. matsui@fukuoka-u.ac.jp
- 2Department of Medicine, Shiga University of Medical Science, Otsu, Japan.
- KMID: 2417674
- DOI: http://doi.org/10.5217/ir.2018.16.2.223
Abstract
- BACKGROUND/AIMS
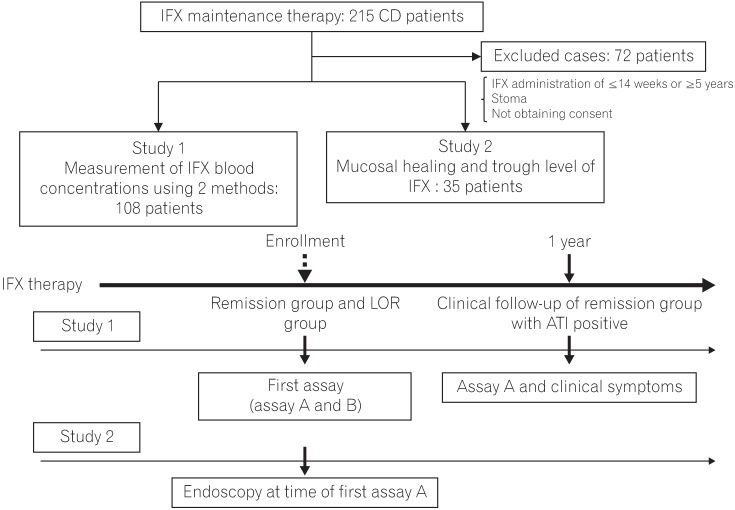
Decreased trough levels of infliximab (TLI) and antibodies to infliximab (ATI) are associated with loss of response (LOR) in Crohn's disease. Two prospective studies were conducted to determine whether TLI or ATI better correlates with LOR (Study 1), and whether TLI could become a predictor of mucosal healing (MH) (Study 2).
METHODS
Study 1 was conducted in 108 patients, including those with LOR and remission to compare ATI and TLI in discriminating the 2 conditions based on receiver operating characteristic (ROC) curve analyses. Study 2 involved 35 patients who were evaluated endoscopically.
RESULTS
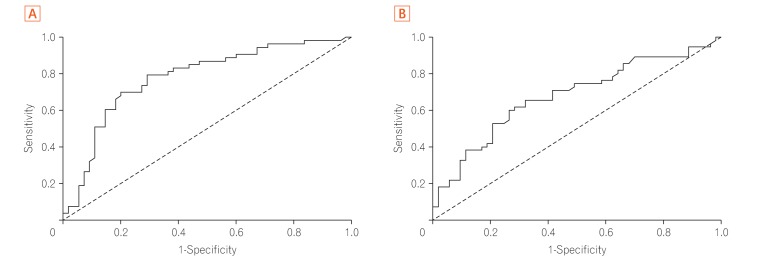
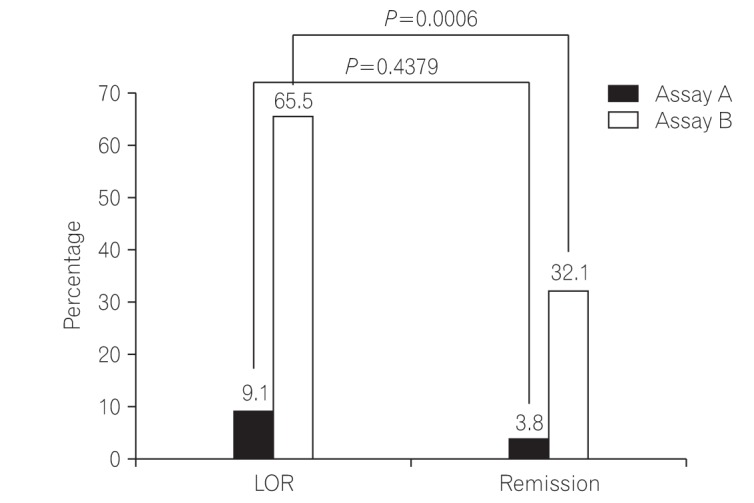
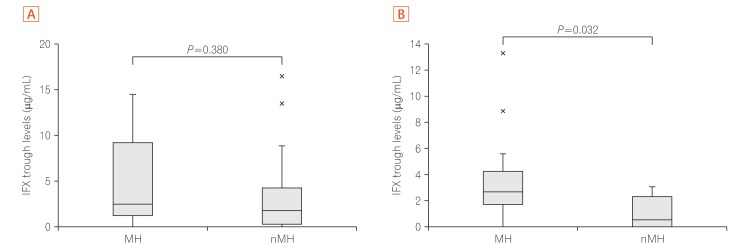
In Study 1, there were no differences between the 2 assays in ROC curve analyses; the TLI cutoff value for LOR was 2.6 µg/mL (sensitivity, 70.9%; specificity, 79.2%), and the ATI cutoff value was 4.9 µg/mL (sensitivity, 65.5%; specificity, 67.9%). The AUROC (area under the ROC curve) of TLI was greater than that of ATI. AUROC was useful for discriminating between the 2 conditions. In Study 2, the TLI was significantly higher in the colonic MH group than in the non-MH group (2.7 µg/mL vs. 0.5 µg/mL, P=0.032).
CONCLUSIONS
TLI is better than ATI for clinically diagnosing LOR, and a correlation was observed between TLI and colonic MH.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Potential Utility of Therapeutic Drug Monitoring of Adalimumab in Predicting Short-Term Mucosal Healing and Histologic Remission in Pediatric Crohn's Disease Patients
So Yoon Choi, Young Ok Choi, Yon Ho Choe, Ben Kang
J Korean Med Sci. 2020;35(16):e114. doi: 10.3346/jkms.2020.35.e114.
Reference
-
1. Gisbert JP, Panés J. Loss of response and requirement of infliximab dose intensification in Crohn's disease: a review. Am J Gastroenterol. 2009; 104:760–767. PMID: 19174781.
Article2. Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn's disease. Aliment Pharmacol Ther. 2011; 33:987–995. PMID: 21366636.
Article3. Danese S, Fiorino G, Reinisch W. Review article: causative factors and the clinical management of patients with Crohn’s disease who lose response to anti-TNF-alpha therapy. Aliment Pharmacol Ther. 2011; 34:1–10. PMID: 21539588.
Article4. Steenholdt C, Bendtzen K, Brynskov J, Thomsen OØ, Ainsworth MA. Cut-off levels and diagnostic accuracy of infliximab trough levels and anti-infliximab antibodies in Crohn's disease. Scand J Gastroenterol. 2011; 46:310–318. PMID: 21087119.
Article5. Vande Casteele N, Khanna R, Levesque BG, et al. The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn’s disease. Gut. 2015; 64:1539–1545. PMID: 25336114.
Article6. Maini RN, Breedveld FC, Kalden JR, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998; 41:1552–1563. PMID: 9751087.
Article7. Imaeda H, Takahashi K, Fujimoto T, et al. Clinical utility of newly developed immunoassays for serum concentrations of adalimumab and anti-adalimumab antibodies in patients with Crohn’s disease. J Gastroenterol. 2014; 49:100–109. PMID: 23575576.
Article8. Imaeda H, Andoh A, Fujiyama Y. Development of a new immunoassay for the accurate determination of anti-infliximab antibodies in inflammatory bowel disease. J Gastroenterol. 2012; 47:136–143. PMID: 21953314.
Article9. Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976; 70:439–444. PMID: 1248701.10. Sou S, Matsui T, Yao T, et al. Clinical and endoscopic healing after infliximab treatment in patients with Crohn’s disease. Dig Endosc. 2006; 18:29–33.
Article11. Beppu T, Ono Y, Matsui T, et al. Mucosal healing of ileal lesions is associated with long-term clinical remission after infliximab maintenance treatment in patients with Crohn’s disease. Dig Endosc. 2015; 27:73–81. PMID: 24833527.
Article12. Imaeda H, Bamba S, Takahashi K, et al. Relationship between serum infliximab trough levels and endoscopic activities in patients with Crohn’s disease under scheduled maintenance treatment. J Gastroenterol. 2014; 49:674–682. PMID: 23666424.
Article13. Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis. 2009; 15:1295–1301. PMID: 19340881.
Article14. Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002; 359:1541–1549. PMID: 12047962.
Article15. Ungar B, Levy I, Yavne Y, et al. Optimizing anti-TNF-alpha therapy: serum levels of infliximab and adalimumab are associated with mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2016; 14:550–557. PMID: 26538204.16. Roblin X, Marotte H, Leclerc M, et al. Combination of C-reactive protein, infliximab trough levels, and stable but not transient antibodies to infliximab are associated with loss of response to infliximab in inflammatory bowel disease. J Crohns Colitis. 2015; 9:525–531. PMID: 25895875.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Early Infliximab Trough Levels Predict the Long-term Efficacy of Infliximab in a Randomized Controlled Trial in Patients with Active Crohn’s Disease Comparing, between CT-P13 and Originator Infliximab
- Factors Affecting Surgical Treatment With Infliximab Therapy in Perianal Fistula With Crohn Disease
- Recent Trends of Infliximab Treatment for Crohn's Disease
- Adalimumab or infliximab: which is better for perianal fistula in Crohn's disease?
- A Case of Infliximab-induced Psoriasis in Treatment of Ankylosing Spondylitis