J Vet Sci.
2018 Jul;19(4):492-499. 10.4142/jvs.2018.19.4.492.
Establishment and identification of cell lines from type O blood Korean native pigs and their efficiency in supporting embryonic development via somatic cell nuclear transfer
- Affiliations
-
- 1Department of Theriogenology and Biotechnology, College of Veterinary Medicine, Seoul National University, Seoul 08826, Korea. clee@snu.ac.kr
- 2Institutes of Green Bio Science and Technology, Seoul National University, Pyeongchang 25354, Korea.
- KMID: 2417563
- DOI: http://doi.org/10.4142/jvs.2018.19.4.492
Abstract
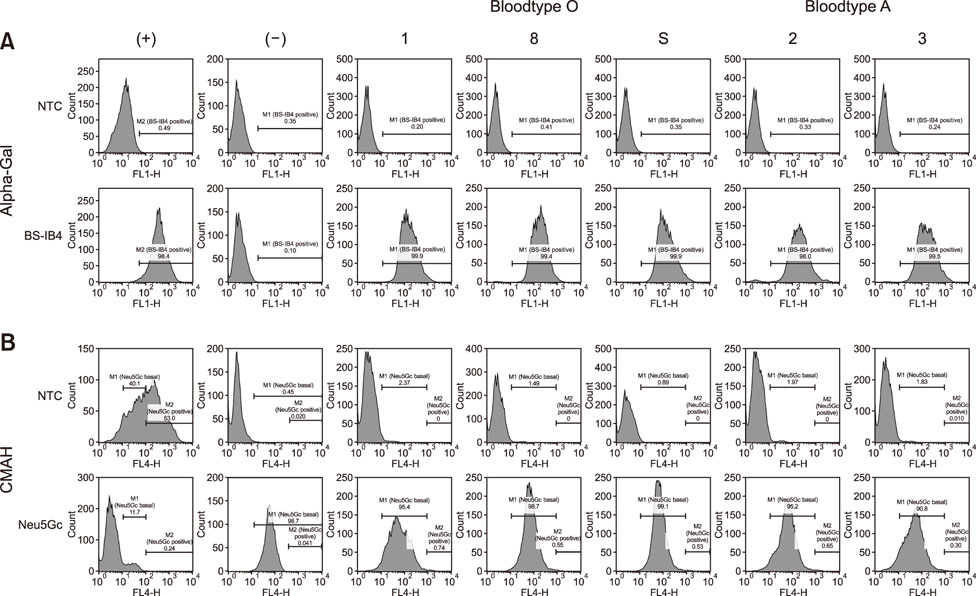
- Due to their similarities with humans in anatomy, physiology, and genetics miniature pigs are becoming an attractive model for biomedical research. We aim to establish and evaluate blood type O cells derived from Korean native pig (KNP), a typical miniature pig breed in Korea. Ten cell lines derived from 8 KNP piglets and one adult female KNP (kidney and ear tissues) were established. To confirm the presence of blood type O, genomic DNA, fucosyltransferase (FUT) expression, and immunofluorescence staining were examined. Additionally, fluorescence-activated cell sorting and somatic cell nuclear transfer were performed to investigate the normality of the cell lines and to evaluate their effectiveness in embryo development. We found no significant bands corresponding to specific blood group A, and no increase in FUT expression in cell lines derived from piglets No. 1, No. 4, No. 5, No. 8, and the adult female KNP; moreover, they showed normal levels of expression of α 1,3-galactosyltransferase and cytidine monophosphate-N-acetylneuraminic acid hydroxylase. There was no significant difference in embryo development between skin and kidney fibroblasts derived from the blood type O KNPs. In conclusion, we successfully established blood type O KNP cell lines, which may serve as a useful model in xenotransplantation research.
MeSH Terms
Figure
Reference
-
1. Cho SK, Hwang KC, Choi YJ, Bui HT, Nguyen VT, Park C, Kim JH, Kim JH. Production of transgenic pigs harboring the human erythropoietin (hEPO) gene using somatic cell nuclear transfer. J Reprod Dev. 2009; 55:128–136.
Article2. Dor FJ, Cheng J, Alt A, Cooper DK, Schuurman HJ. Galα1,3Gal expression on porcine pancreatic islets, testis, spleen, and thymus. Xenotransplantation. 2004; 11:101–106.
Article3. He J, Li Q, Fang S, Guo Y, Liu T, Ye J, Yu Z, Zhang R, Zhao Y, Hu X, Bai X, Chen X, Li N. PKD1 mono-allelic knockout is sufficient to trigger renal cystogenesis in a mini-pig model. Int J Biol Sci. 2015; 11:361–369.
Article4. Hur SJ, Jeong TC, Kim GD, Jeong JY, Cho IC, Lim HT, Kim BW, Joo ST. Comparison of live performance and meat quality parameter of cross bred (Korean native black pig and landrace) pigs with different coat colors. Asian-Australas J Anim Sci. 2013; 26:1047–1053.
Article5. Hwang IS, Kwon DJ, Oh KB, Ock SA, Chung HJ, Cho IC, Lee JW, Im GS, Hwang S. Production of cloned Korean native pig by somatic cell nuclear transfer. Dev Reprod. 2015; 19:79–84.
Article6. Kim GA, Lee EM, Jin JX, Lee S, Taweechaipaisankul A, Hwang JI, Alam Z, Ahn C, Lee BC. Generation of CMAHKO/GTKO/shTNFRI-Fc/HO-1 quadruple gene modified pigs. Transgenic Res. 2017; 26:435–445.
Article7. Kim KS, Yeo JS, Kim JW. Assessment of genetic diversity of Korean native pig (Sus scrofa) using AFLP markers. Genes Genet Syst. 2002; 77:361–368.
Article8. Kim S, Kim JH, Lee E, Jeong YW, Hossein MS, Park SM, Park SW, Lee JY, Jeong YI, Kim HS, Kim YW, Hyun SH, Hwang WS. Establishment and characterization of embryonic stem-like cells from porcine somatic cell nuclear transfer blastocysts. Zygote. 2010; 18:93–101.
Article9. Kurome M, Ishikawa T, Tomii R, Ueno S, Shimada A, Yazawa H, Nagashima H. Production of transgenic and non-transgenic clones in miniature pigs by somatic cell nuclear transfer. J Reprod Dev. 2008; 54:156–163.
Article10. Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, Samuel M, Bonk A, Rieke A, Day BN, Murphy CN, Carter DB, Hawley RJ, Prather RS. Production of α-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002; 295:1089–1092.
Article11. Lee JB, Jung EJ, Park HB, Jin S, Seo DW, Ko MS, Cho IC, Lee JH, Lim HT. Genome-wide association analysis to identify SNP markers affecting teat numbers in an F2 intercross population between Landrace and Korean native pigs. Mol Biol Rep. 2014; 41:7167–7173.
Article12. Lee KT, Lee YM, Alam M, Choi BH, Park MR, Kim KS, Kim TH, Kim JJ. A whole genome association study on meat quality traits using high density SNP chips in a cross between Korean native pig and Landrace. Asian-Australas J Anim Sci. 2012; 25:1529–1539.
Article13. Lee S, Park EJ, Moon JH, Kim SJ, Song K, Lee BC. Sequential treatment with resveratrol-trolox improves development of porcine embryos derived from parthenogenetic activation and somatic cell nuclear transfer. Theriogenology. 2015; 84:145–154.
Article14. Leight GS, Kirkman R, Rasmusen BA, Rosenberg SA, Sachs DH, Terrill R, Williams GM. Transplantation in miniature swine. III: Effects of MSLA and A-O blood group matching on skin allograft survival. Tissue Antigens. 1978; 12:65–74.
Article15. Lutz AJ, Li P, Estrada JL, Sidner RA, Chihara RK, Downey SM, Burlak C, Wang ZY, Reyes LM, Ivary B, Yin F, Blankenship RL, Paris LL, Tector AJ. Double knockout pigs deficient in N-glycolylneuraminic acid and Galactose α-1,3-Galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation. 2013; 20:27–35.
Article16. McKenzie IFC, Xing PX, Vaughan HA, Prenzoska J, Dabkowski PL, Sandrin MS. Distribution of the major xenoantigen (gal(α1-3)gal) for pig to human xenografts. Transpl Immunol. 1994; 2:81–86.
Article17. Park SJ, Cho B, Koo OJ, Kim H, Kang JT, Hurh S, Kim SJ, Yeom HJ, Moon J, Lee EM, Choi JY, Hong JH, Jang G, Hwang JI, Yang J, Lee BC, Ahn C. Production and characterization of soluble human TNFRI-Fc and human HO-1(HMOX1) transgenic pigs by using the F2A peptide. Transgenic Res. 2014; 23:407–419.
Article18. Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, Ball S, Specht SM, Polejaeva IA, Monahan JA, Jobst PM, Sharma SB, Lamborn AE, Garst AS, Moore M, Demetris AJ, Rudert WA, Bottino R, Bertera S, Trucco M, Starzl TE, Dai Y, Ayares DL. Production of α1,3-galactosyltransferase-deficient pigs. Science. 2003; 299:411–414.
Article19. Rydberg L. ABO-incompatibility in solid organ transplantation. Transfus Med. 2001; 11:325–342.
Article20. Smith DM, Newhouse M, Naziruddin B, Kresie L. Blood groups and transfusions in pigs. Xenotransplantation. 2006; 13:186–194.
Article21. Taweechaipaisankul A, Jin JX, Lee S, Kim GA, Lee BC. The effects of canthaxanthin on porcine oocyte maturation and embryo development in vitro after parthenogenetic activation and somatic cell nuclear transfer. Reprod Domest Anim. 2016; 51:870–876.
Article22. Wang ZY, Burlak C, Estrada JL, Li P, Tector MF, Tector AJ. Erythrocytes from GGTA1/CMAH knockout pigs: implications for xenotransfusion and testing in non-human primates. Xenotransplantation. 2014; 21:376–384.
Article23. Wei H, Qing Y, Pan W, Zhao H, Li H, Cheng W, Zhao L, Xu C, Li H, Li S, Ye L, Wei T, Li X, Fu G, Li W, Xin J, Zeng Y. Comparison of the efficiency of Banna miniature inbred pig somatic cell nuclear transfer among different donor cells. PLoS One. 2013; 8:e57728.24. Yeom HJ, Koo OJ, Yang J, Cho B, Hwang JI, Park SJ, Hurh S, Kim H, Lee EM, Ro H, Kang JT, Kim SJ, Won JK, O'Connell PJ, Kim H, Surh CD, Lee BC, Ahn C. Generation and characterization of human heme oxygenase-1 transgenic pigs. PLoS One. 2012; 7:e46646.
Article25. Yip SP, Chee KY, Chan PY, Chow EY, Wong HF. Molecular genetic analysis of para-Bombay phenotypes in Chinese: a novel non-functional FUT1 allele is identified. Vox Sang. 2002; 83:258–262.
Article26. Zhao J, Ross JW, Hao Y, Spate LD, Walters EM, Samuel MS, Rieke A, Murphy CN, Prather RS. Significant improvement in cloning efficiency of an inbred miniature pig by histone deacetylase inhibitor treatment after somatic cell nuclear transfer. Biol Reprod. 2009; 81:525–530.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of porcine urine-derived cells as nuclei donor for somatic cell nuclear transfer
- Expression of polo-like kinase 1 in pre-implantation stage murine somatic cell nuclear transfer embryos
- Developmental competence of chimeric porcine embryos through the aggregation of parthenogenetic embryos and somatic cell nuclear transfer embryos
- Delivering Factors for Reprogramming a Somatic Cell to Pluripotency
- In vitro maturation using αMEM with reduced NaCl enhances maturation and developmental competence of pig oocytes after somatic cell nuclear transfer