Nat Prod Sci.
2018 Jun;24(2):88-92. 10.20307/nps.2018.24.2.88.
Garcinexanthone G, a Selective Butyrylcholinesterase Inhibitor from the Stem Bark of Garcinia atroviridis
- Affiliations
-
- 1Discipline of Pharmacology, School of Pharmaceutical Sciences, Universiti Sains Malaysia, 11800 Penang, Malaysia.
- 2School of Chemical Sciences, Universiti Sains Malaysia, 11800 Penang, Malaysia.
- 3School of Distance Education, Universiti Sains Malaysia, 11800 Penang, Malaysia. tanwn@usm.my
- KMID: 2416067
- DOI: http://doi.org/10.20307/nps.2018.24.2.88
Abstract
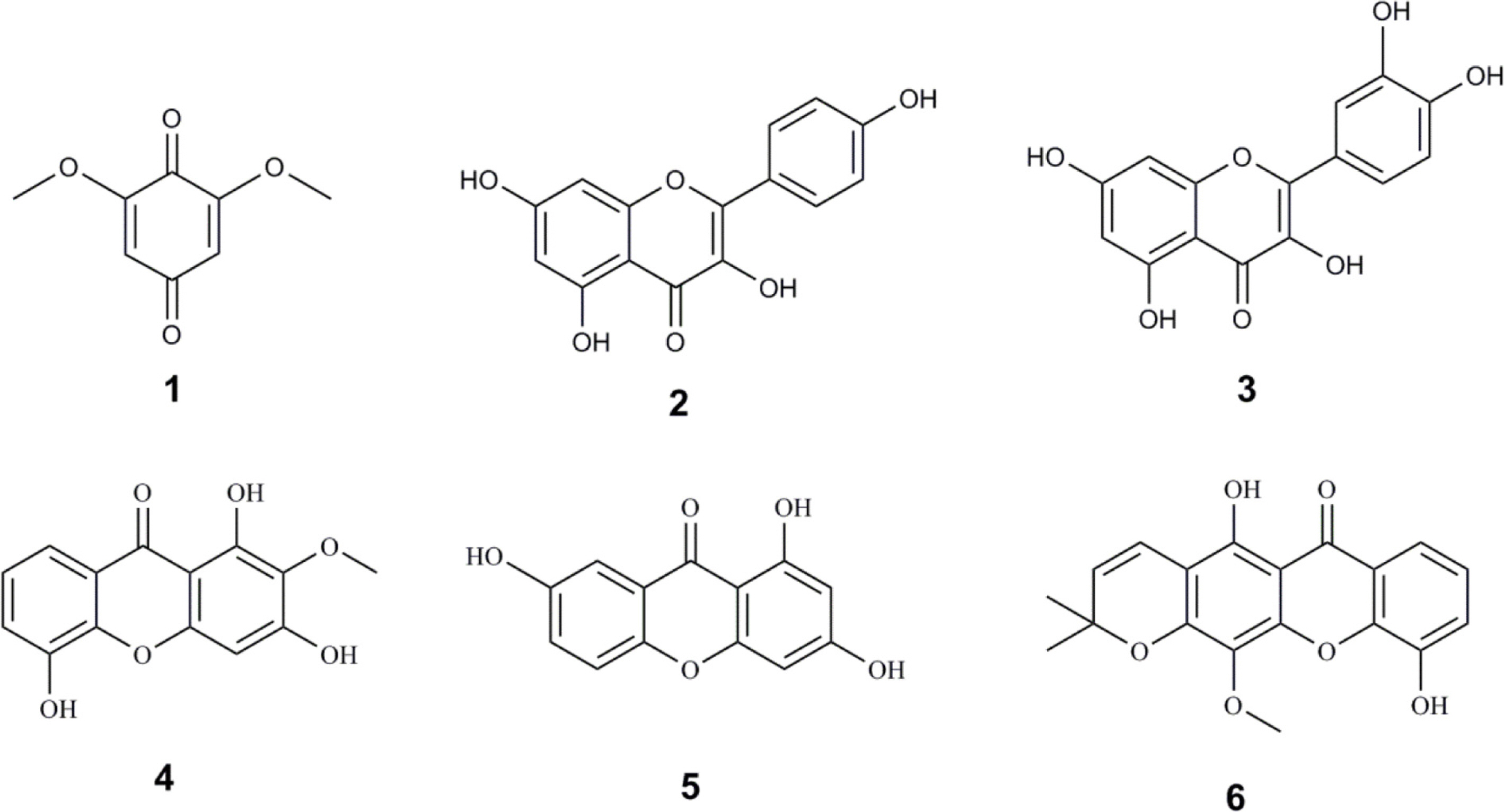
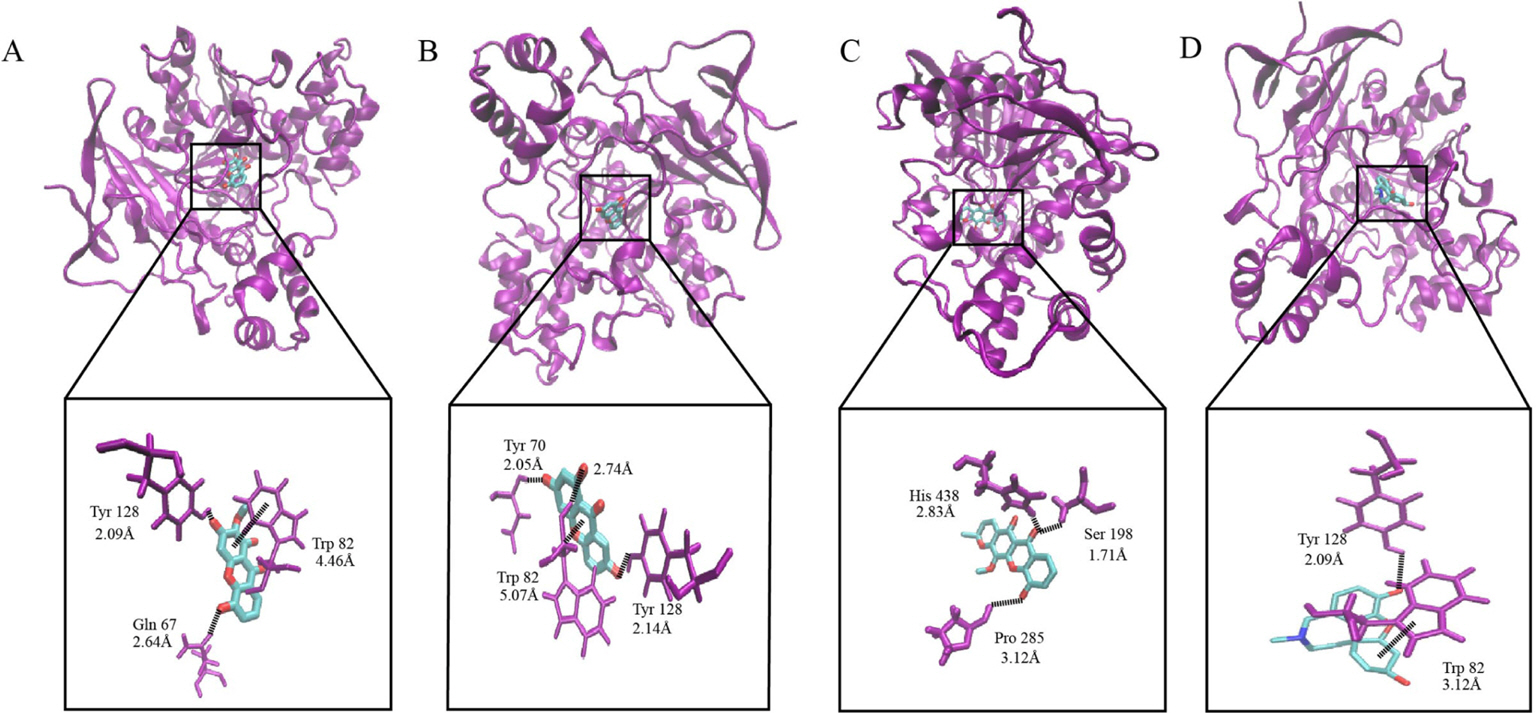
- The present study was undertaken to investigate the isolated compounds from the stem bark of Garcinia atroviridis as potential cholinesterase inhibitors and the ligand-enzyme interactions of selected bioactive compounds in silico. The in vitro cholinesterase results showed that quercetin (3) was the most active AChE inhibitor (12.65 ± 1.57 µg/ml) while garcinexanthone G (6) was the most active BChE inhibitor (18.86 ± 2.41 µg/ml). It is noteworthy to note that compound 6 was a selective inhibitor with the selectivity index of 11.82. Molecular insight from docking interaction further substantiate that orientation of compound 6 in the catalytic site which enhanced its binding affinity as compared to other xanthones. The nature of protein-ligand interactions of compound 6 is mainly hydrogen bonding, and the hydroxyl group of compound 6 at C-10 is vital in BChE inhibition activity. Therefore, compound 6 is a notable lead for further drug design and development of BChE selective inhibitor.
MeSH Terms
Figure
Reference
-
References
(1). Tarawneh R.., Holtzman D. M.Cold Spring Harb. Perspect. Med. 2012. 2:, a006148.(2). Prince M.., Comas-Herrera A.., Knapp M.., Guerchet M.., Karagiannidou M.World Alzheimer Report. 2016.(3). Herrup K.Nat. Neurosci. 2015. 18:794–799.(4). Aguzzi A.., O'Connor T.Nat. Rev. Drug Discov. 2010. 9:237–248.(5). Hardy J.., Selkoe D. J.Science. 2002. 297:353–356.(6). Colovi M. B.., Krsti D. Z.., Lazarevi -Pašti T. D.., Bondži A. có có có có M.., Vasi V. M.Curr. Neuropharmacol. 2013. 11:315–335.(7). Scarpini E.., Schelterns P.., Feldman H.Lancet Neurol. 2003. 2:539–547.(8). Darvesh S.., Grantham D. L.., Hopkins D. A. J.Comp. Neurol. 1998. 393:374–390.(9). Perry E. K.., Perry R. H.., Blessed G.., Tomlinson B. E.Neuropathol. Appl. Neurobiol. 1978. 4:273–277.(10). Quinn D. M.Chem. Rev. 1987. 87:955–979.
Article(11). Sussman J. L.., Harel M.., Frolow F.., Oefner C.., Goldman A.., Toker L.., Silman I.Science. 1991. 253:872–879.(12). Greig N. H.., Utsuki T.., Ingram D. K.., Wang Y.., Pepeu G.., Scali C.., Yu Q. S.., Mamczarz J.., Holloway H. W.., Giordano T.., Chen D.., Furukawa K.., Sambamurti K.., Brossi A.., Lahiri D. K. Proc. Natl. Acad. Sci. U. S.A. 2005. 102:17213–17218.(13). Tan W. N.., Khairuddean M.., Wong K. C.., Khaw K. Y.., Vikneswaran M.Fitoterapia. 2014. 97:261–267.(14). Tan W. N.., Khairuddean M.., Wong K. C.., Tong W. Y.., Ibrahim D. J.Asian Nat. Prod. Res. 2016. 18:804–811.(15). Ellman G. L.., Courtney K. D.., Andres V. Jr.., Feather-Stone R. M.Biochem. Pharmacol. 1961. 7:88–95.(16). Morris G. M.., Goodsell D. S.., Halliday R. S.., Huey R.., Hart W. E.., Belew R. K.., Olson A. J. J.Comput. Chem. 1998. 19:1639–1662.(17). Carletti E.., Aurbek N.., Gillon E.., Loiodice M.., Nicolet Y.., Fontecilla-Camps J. -C.., Masson P.., Thiermann H.., Nachon F.., Worek F.Biochem. J. 2009. 421:97–106.(18). Humphrey W.., Dalke A.., Schulten K. J.Mol. Graph. 1996. 14:33–38.(19). Khaw K. Y.., Choi S. B.., Tan S. C.., Wahab H. A.., Chan K. L.., Murugaiyah V.Phytomedicine. 2014. 21:1303–1309.(20). Louh G. N.., Lannang A. M.., Mbazoa C. D.., Tangmouo J. G.., Komguem J.., Castilho P.., Ngninzeko F. N.., Qamar N.., Lontsi D.., Choudhary M. I.., Sondengam B. L.Phytochemistry. 2008. 69:1013–1017.(21). Khan M. T. H.., Orhan I.., Senol F. S.., Kartal M.., Sener B.., Dvorská M.., Smejkal K.., Slapetová T.Chem. Biol. Interact. 2009. 181:383–389.(22). Sriraksa N.., Wattanathorn J.., Muchimapura S.., Tiamkao S.., Brown K.., Chaisiwamongkol K.Evid. Based Complement. Alternat. Med. 2012. 2012:823206.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Butyrylcholinesterase deficiency identified by preoperative patient interview
- Parvidepsidone, a Novel Depsidone from the Barks of Garcinia parvifolia Miq.
- Sesbagrandiflorain F, a New 2-Arylbenzofuran from the Stem Bark of Sesbania grandiflora L.
- Sesquiterpenoids from the heartwood of Juniperu s chinensis
- Inhibition of Class I Histone Deacetylase Enhances Self-Reprogramming of Spermatogonial Stem Cells into Pluripotent Stem Cells