Nat Prod Sci.
2017 Sep;23(3):208-212. 10.20307/nps.2017.23.3.208.
Sesquiterpenoids from the heartwood of Juniperu s chinensis
- Affiliations
-
- 1Department of Food and Life Science, Pukyong National University, Busan 608-737, Republic of Korea. choijs@pknu.ac.kr
- 2College of Pharmacy, Catholic University of Daegu, Gyeongbuk 712-702, Republic of Korea.
- 3Department of Food Science and Human Nutrition, Chonbuk National University, Jeonju 561-756, Republic of Korea. jungha@jbnu.ac.kr
- KMID: 2393802
- DOI: http://doi.org/10.20307/nps.2017.23.3.208
Abstract
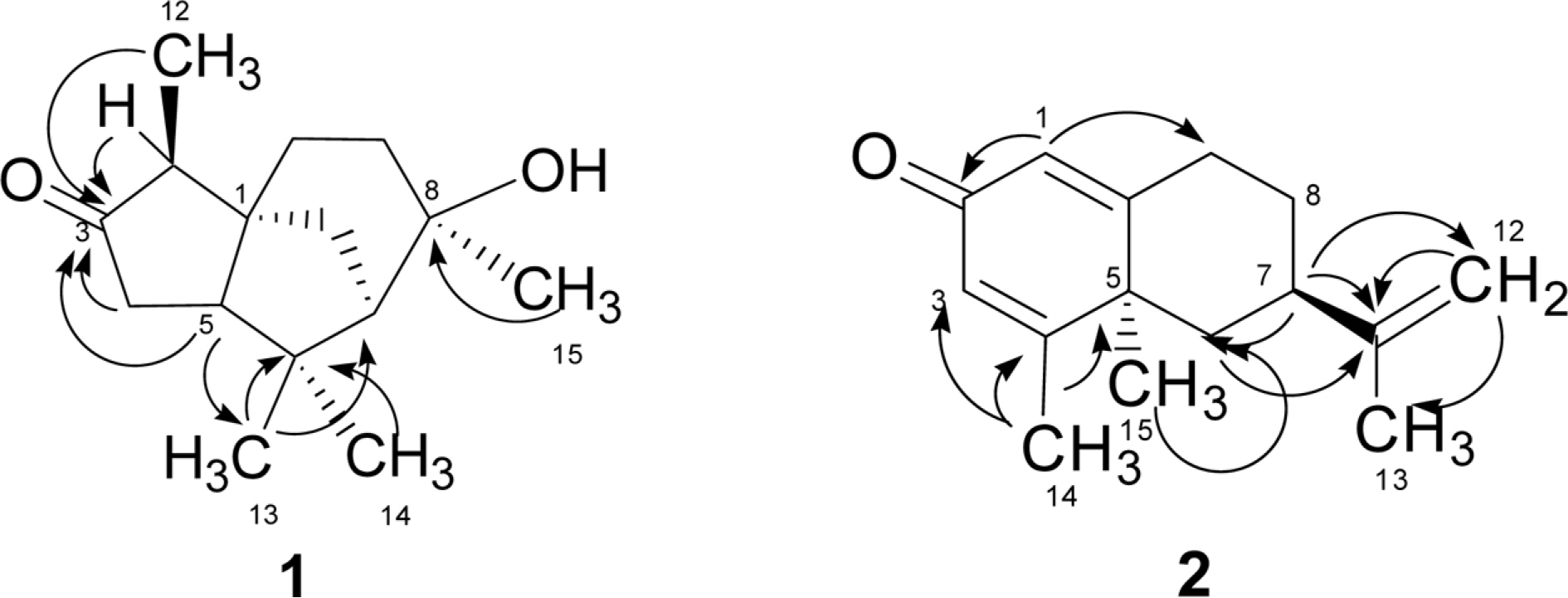
- A new sesquiterpenoid, 11-hydroxy-valenc-1(10),3(4)-dien-2-one (3), two chemically synthesized but first isolate from nature, 3-oxocedran-8β-ol (1) and valenc-1(10),3(4),11(12)-trien-2-one (2) along with four known compounds, sugiol (4), (+)-nootkatone (5), 11-hydroxy-valenc-1(10)-en-2-one (6), and clovandiol (7), were isolated from the heartwood of Juniperus chinensis. All chemical structures were elucidated using extensive spectroscopic analysis including 1D and 2D NMR spectroscopy. Valenc-1(10),3(4),11(12)-trien-2-one (2) exhibited significant inhibitory activity against butyrylcholinesterase with an ICâ‚…â‚€ value of 68.45 µM.
Keyword
MeSH Terms
Figure
Reference
-
References
(1). Corner E. J. H.Wayside Trees of Malaya; Malayan Nature Society: Malaysia,. 1988. 396–446.(2). But P. P. H.., Kimura T.., Gue J. X.., Sung C. K.International Collation of Traditional and Folk Medicine; World Scientific Publishing Co., Ltd.: Singapore,. 1997. 16–17.(3). Jung H. J.., Jung H. A.., Min B. S.., Choi J. S.Chem. Pharm. Bull. 2015. 63:955–960.(4). Ellman G. L.., Courtney K. D.., Andres V. Jr.., Feather-stone R. M.Biochem. Pharmacol. 1961. 7:88–95.(5). Hanson J. R.., Nasir H.Phytochemistry. 1993. 33:835–837.(6). Gand E.., Hanson J. R.., Nasir H.Phytochemistry. 1995. 39:1081–1084.(7). Collins D. O.., Reese P. B.Phytochemistry. 2001. 56:417–421.(8). Caine D.., Chu C. Y.Tetrahedron Lett. 1974. 15:703–706.(9). Caine D.., Chu C. Y.., Graham S. L. J.Org. Chem. 1980. 45:3790–3797.(10). Pesaro M.., Bozzato G.., Schudel P.Chem. Commun (London). 1968. 19:1152–1154.(11). Kuo Y. H.., Wu T. R.., Cheng M. C.., Wang Y.Chem. Pharm. Bull. 1990. 38:3195–3201.(12). Chang C. I.., Chen W. C.., Shao Y. Y.., Yeh C. R.., Yang N. S.., Chiang W.., Kuo Y. H.Nat. Prod. Res. 2008. 22:1158–1162.(13). Savona G.., Piozzi F.., De La Torre M. C.., Servettaz, O. Rodríguez B.Phytochemistry. 1987. 26:571–572.(14). Xu J.., Su J.., Li Y.., Tan N.Chem. Nat. Compd. 2013. 49:457–461.(15). Zhang C.., Zhou A.., Zhang M.China Journal of Chinese Materia Medica. 2009. 34:994–998.(16). Furusawa M.., Hashimoto T.., Noma Y.., Asakawa Y.Chem. Pharm. Bull. 2005. 53:1513–1514.(17). Naf F.., Decorzant R.., Thommen W.Helv. Chim. Acta. 1982. 65:2212–2223.(18). Wittayalai S.., Mahidol C.., Prachyawarakorn V.., Prawat H.., Ruchirawat S.Phytochemistry. 2014. 99:121–126.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibitory effects of Coptis chinensis extract on the growth and biofilm formation of Streptococcus mutans and Streptococcus sobrinus
- Cytotoxic Neoflavonoids and Chalcones from the Heartwood of Dalbergia melanoxylon
- Inhibition of Interleukin-4 and β-Hexosaminidase Release in RBL-2H3 Cells by Compounds Isolated from Lobelia chinensis
- Sesquiterpenoids from the Stem Bark of Aglaia grandis
- A Case of Ant Sting By Brachyponera chinensis