Yonsei Med J.
2018 Aug;59(6):746-753. 10.3349/ymj.2018.59.6.746.
mRNA Expression of SLC5A5 and SLC2A Family Genes in Papillary Thyroid Cancer: An Analysis of The Cancer Genome Atlas
- Affiliations
-
- 1Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea.
- 2Department of Anatomy, School of Medicine, Pusan National University, Yangsan, Korea.
- 3Department of Orthopaedic Surgery and Biomedical Research Institute, Pusan National University Hospital, Busan, Korea. taesikgoh@pusan.ac.kr
- 4Department of Statistics, Korea University, Seoul, Korea.
- 5Biomedical Research Institute, Pusan National University Hospital and School of Medicine, Pusan National University, Busan, Korea.
- 6Department of Otorhinolaryngology-Head and Neck Surgery, Ajou University Hospital, Suwon, Korea.
- 7Department of Otorhinolaryngology-Head and Neck Surgery, Pusan National University Hospital, Busan, Korea.
- 8Department of Nuclear Medicine and Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Yangsan, Korea.
- 9Department of Nuclear Medicine and Biomedical Research Institute, Pusan National University Hospital, Busan, Korea. ilikechopin@me.com
- KMID: 2415533
- DOI: http://doi.org/10.3349/ymj.2018.59.6.746
Abstract
- PURPOSE
The present study investigated the dynamics and prognostic role of messenger RNA (mRNA) expression responsible for 18F-fluorodeoxyglucose (FDG) uptake in FDG positron emission tomography (PET) and radioactive iodine (131I) uptake in whole-body radioactive iodine scans (WBS) in papillary thyroid cancer (PTC) patients.
MATERIALS AND METHODS
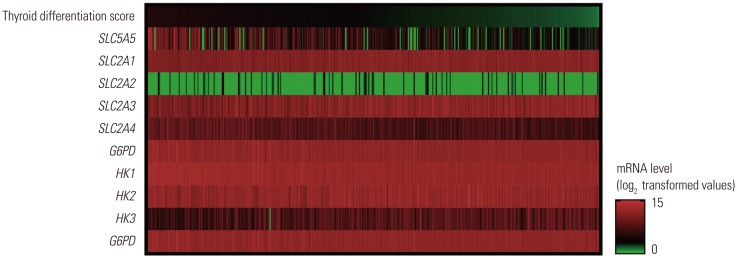
The primary and processed data were downloaded from the Genomic Data Commons Data Portal. Expression data for sodium/iodide symporter (solute carrier family 5 member 5, SLC5A5), hexokinase (HK1-3), glucose-6-phosphate dehydrogenase (G6PD), and glucose transporter (solute carrier family 2, SLC2A1-4) mRNA were collected.
RESULTS
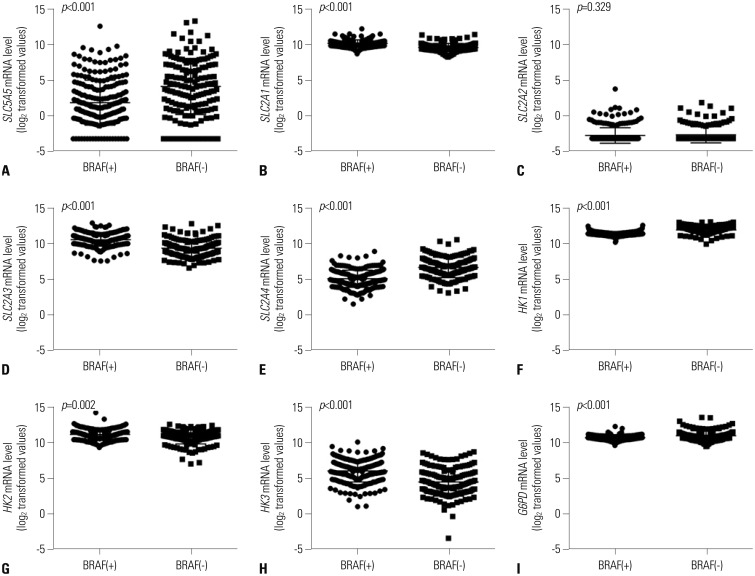
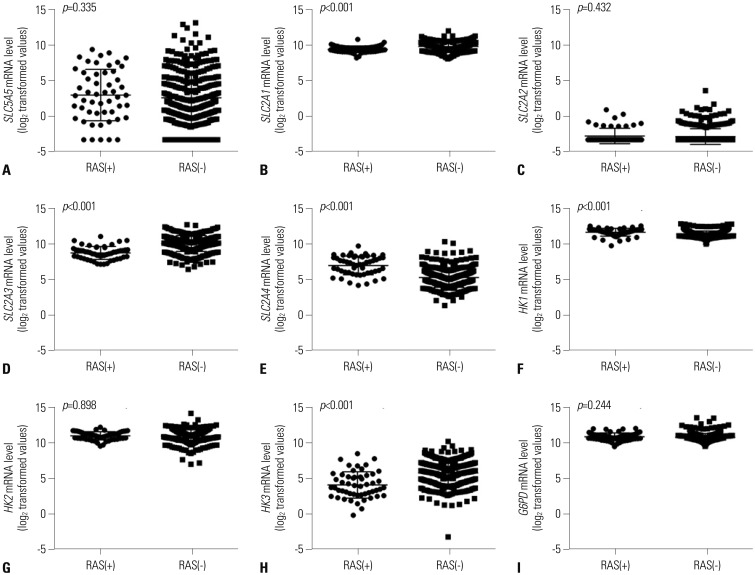
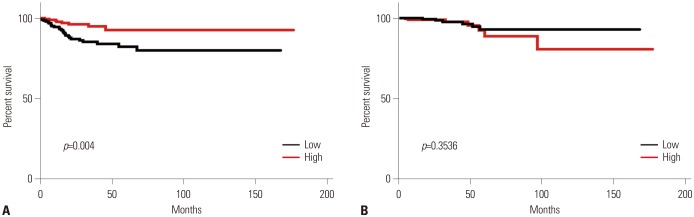
Expression of SLC5A5 mRNA were negatively correlated with SLC2A1 mRNA and positively correlated with SLC2A4 mRNA. In PTC with BRAF mutations, expressions of SLC2A1, SLC2A3, HK2, and HK3 mRNA were higher than those in PTC without BRAF mutations. Expression of SLC5A5, SLC2A4, HK1, and G6PD mRNA was lower in PTC without BRAF mutation. PTCs with higher expression of SLC5A5 mRNA had more favorable disease-free survival, but no association with overall survival.
CONCLUSION
Expression of SLC5A5 mRNA was negatively correlated with SLC2A1 mRNA. This finding provides a molecular basis for the management of PTC with negative WBS using 18F-FDG PET scans. In addition, higher expression of SLC5A5 mRNA was associated with less PTC recurrence, but not with deaths.
MeSH Terms
-
Disease-Free Survival
Fluorodeoxyglucose F18
Genome*
Glucose Transport Proteins, Facilitative
Glucosephosphate Dehydrogenase
Hexokinase
Humans
Iodine
Ion Transport
Positron-Emission Tomography
Recurrence
RNA, Messenger*
Thyroid Gland*
Thyroid Neoplasms*
Fluorodeoxyglucose F18
Glucose Transport Proteins, Facilitative
Glucosephosphate Dehydrogenase
Hexokinase
Iodine
RNA, Messenger
Figure
Reference
-
1. Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. 2016; 388:2783–2795. PMID: 27240885.
Article2. Ahn HS, Welch HG. South Korea's thyroid-cancer “Epidemic”--turning the tide. N Engl J Med. 2015; 373:2389–2390. PMID: 26650173.3. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133. PMID: 26462967.
Article4. Pak K, Cheon GJ, Nam HY, Kim SJ, Kang KW, Chung JK, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: a systematic review and meta-analysis. J Nucl Med. 2014; 55:884–890. PMID: 24752671.
Article5. Tavares C, Melo M, Cameselle-Teijeiro JM, Soares P, Sobrinho-Simöes M. ENDOCRINE TUMOURS: Genetic predictors of thyroid cancer outcome. Eur J Endocrinol. 2016; 174:R117–R126. PMID: 26510840.
Article6. Chai YJ, Yi JW, Oh SW, Kim YA, Yi KH, Kim JH, et al. Upregulation of SLC2 (GLUT) family genes is related to poor survival outcomes in papillary thyroid carcinoma: analysis of data from The Cancer Genome Atlas. Surgery. 2017; 161:188–194. PMID: 27842912.
Article7. Smith TA. FDG uptake, tumour characteristics and response to therapy: a review. Nucl Med Commun. 1998; 19:97–105. PMID: 9548192.8. Filetti S, Bidart JM, Arturi F, Caillou B, Russo D, Schlumberger M. Sodium/iodide symporter: a key transport system in thyroid cancer cell metabolism. Eur J Endocrinol. 1999; 141:443–457. PMID: 10576759.
Article9. Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014; 159:676–690. PMID: 25417114.10. Ros P, Rossi DL, Acebrón A, Santisteban P. Thyroid-specific gene expression in the multi-step process of thyroid carcinogenesis. Biochimie. 1999; 81:389–396. PMID: 10401674.
Article11. Winter J, Winter M, Krohn T, Heinzel A, Behrendt FF, Tuttle RM, et al. Patients with high-risk differentiated thyroid cancer have a lower I-131 ablation success rate than low-risk ones in spite of a high ablation activity. Clin Endocrinol (Oxf). 2016; 85:926–931. PMID: 27256714.
Article12. Ravazoula P, Batistatou A, Aletra C, Ladopoulos J, Kourounis G, Tzigounis B. Immunohistochemical expression of glucose transporter Glut1 and cyclin D1 in breast carcinomas with negative lymph nodes. Eur J Gynaecol Oncol. 2003; 24:544–546. PMID: 14658600.13. Haber RS, Weiser KR, Pritsker A, Reder I, Burstein DE. GLUT1 glucose transporter expression in benign and malignant thyroid nodules. Thyroid. 1997; 7:363–367. PMID: 9226204.
Article14. Józ´wiak P, Krzes´lak A, Pomorski L, Lipin´ ska A. Expression of hypoxia-related glucose transporters GLUT1 and GLUT3 in benign, malignant and non-neoplastic thyroid lesions. Mol Med Rep. 2012; 6:601–606. PMID: 22752218.15. Kim S, Chung JK, Min HS, Kang JH, Park DJ, Jeong JM, et al. Expression patterns of glucose transporter-1 gene and thyroid specific genes in human papillary thyroid carcinoma. Nucl Med Mol Imaging. 2014; 48:91–97. PMID: 24900148.
Article16. Chung JK. Sodium iodide symporter: its role in nuclear medicine. J Nucl Med. 2002; 43:1188–1200. PMID: 12215558.17. Moon SH, Oh YL, Choi JY, Baek CH, Son YI, Jeong HS, et al. Comparison of 18F-fluorodeoxyglucose uptake with the expressions of glucose transporter type 1 and Na+/I− symporter in patients with untreated papillary thyroid carcinoma. Endocr Res. 2013; 38:77–84. PMID: 22888973.18. Chung JK, So Y, Lee JS, Choi CW, Lim SM, Lee DS, et al. Value of FDG PET in papillary thyroid carcinoma with negative 131I whole-body scan. J Nucl Med. 1999; 40:986–992. PMID: 10452315.19. Morari EC, Marcello MA, Guilhen AC, Cunha LL, Latuff P, Soares FA, et al. Use of sodium iodide symporter expression in differentiated thyroid carcinomas. Clin Endocrinol (Oxf). 2011; 75:247–254. PMID: 21521301.
Article20. Yamamoto T, Seino Y, Fukumoto H, Koh G, Yano H, Inagaki N, et al. Over-expression of facilitative glucose transporter genes in human cancer. Biochem Biophys Res Commun. 1990; 170:223–230. PMID: 2372287.
Article21. Pak K, Suh S, Kim SJ, Kim IJ. Prognostic value of genetic mutations in thyroid cancer: a meta-analysis. Thyroid. 2015; 25:63–70. PMID: 25244593.
Article22. Suh S, Pak K, Seok JW, Kim IJ. Prognostic value of extranodal extension in thyroid cancer: a meta-analysis. Yonsei Med J. 2016; 57:1324–1328. PMID: 27593858.
Article23. Ward LS, Santarosa PL, Granja F, da AssumpÇão LV, Savoldi M, Goldman GH. Low expression of sodium iodide symporter identifies aggressive thyroid tumors. Cancer Lett. 2003; 200:85–91. PMID: 14550956.
Article24. Schlumberger MJ. Papillary and follicular thyroid carcinoma. N Engl J Med. 1998; 338:297–306. PMID: 9445411.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Sodium-Iodide Symporter Depending on Mutational Status and Lymphocytic Thyroiditis in Papillary Thyroid Carcinoma
- Genomic Characterization of Differentiated Thyroid Carcinoma
- Expression of NF2 Modulates the Progression of BRAFV600E Mutated Thyroid Cancer Cells
- SOX12 Promotes Thyroid Cancer Cell Proliferation and Invasion by Regulating the Expression of POU2F1 and POU3F1
- In silico Identification of SFRP1 as a Hypermethylated Gene in Colorectal Cancers