Blood Res.
2018 Mar;53(1):53-60. 10.5045/br.2018.53.1.53.
Investigation of BAX and BCL2 expression and apoptosis in a resveratrol- and prednisolone-treated human T-ALL cell line, CCRF-CEM
- Affiliations
-
- 1Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran.
- 2Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran. m.farshdousti@gmail.com
- 3Hematology and Oncology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
- 4Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
- 5Department of Biochemistry, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran.
- 6Department of Basic Sciences, Maragheh University of Medical Sciences, Maragheh, Iran.
- KMID: 2414365
- DOI: http://doi.org/10.5045/br.2018.53.1.53
Abstract
- BACKGROUND
The numerous side effects and chemo-resistance of conventional chemical drugs in the treatment of malignancies have led to consideration of the anti-cancer properties of natural products. In the present study, we aimed to explore the apoptotic effect of two natural components, resveratrol and prednisolone, on the T acute lymphoblastic leukemia (ALL) cell line, CCRF-CEM. Our findings suggested the incorporation of these natural agents into drug regimens to treat patients with ALL.
METHODS
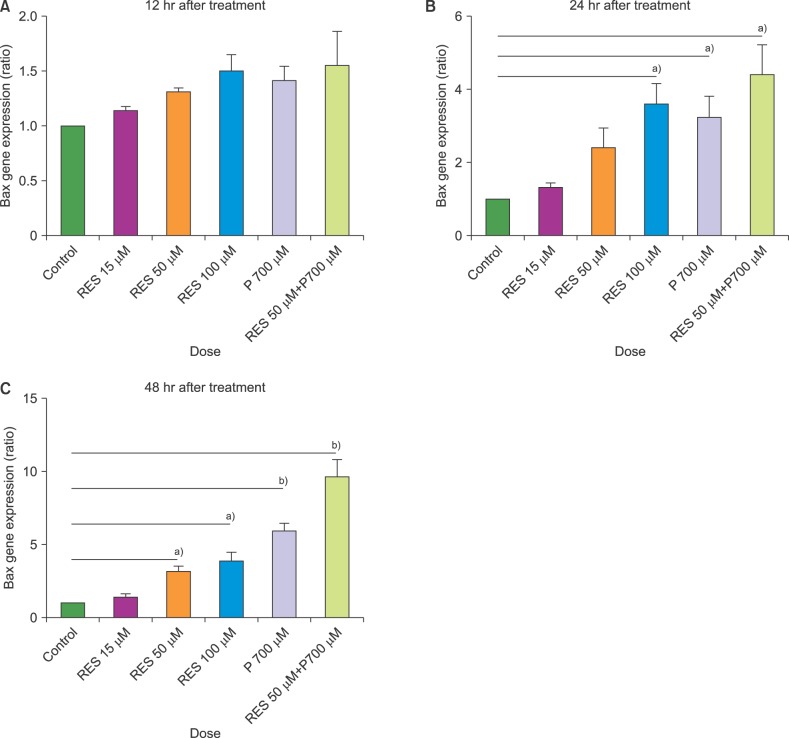
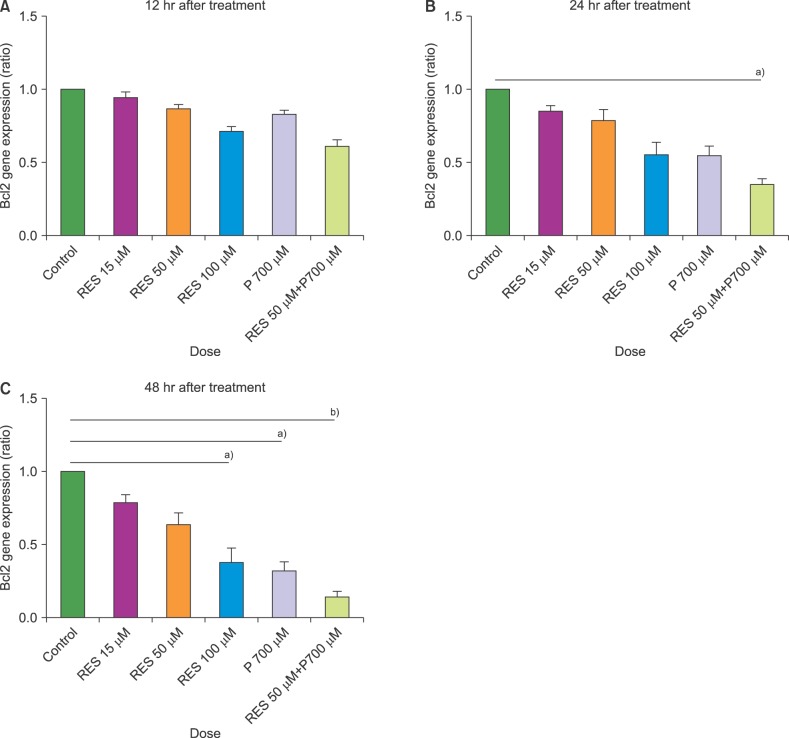
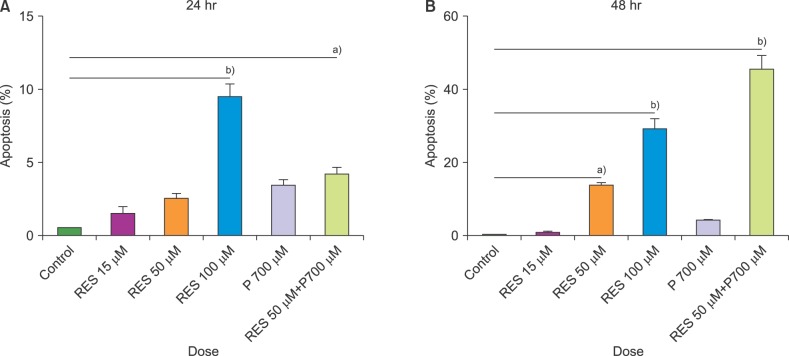
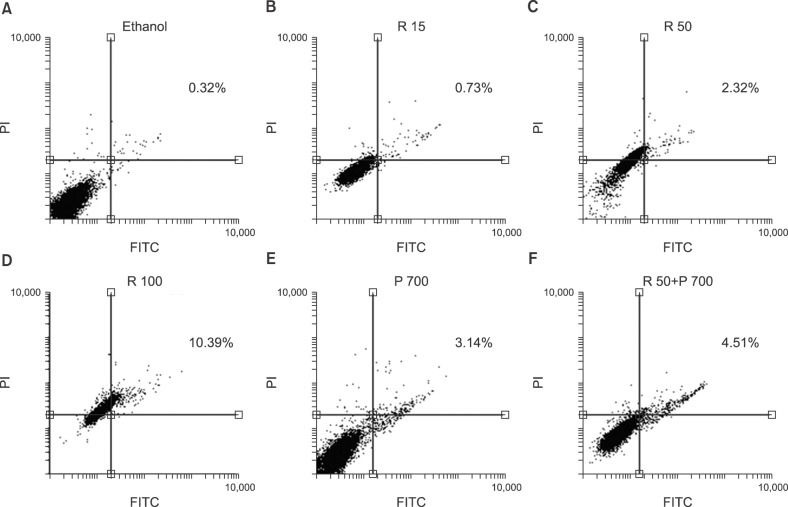
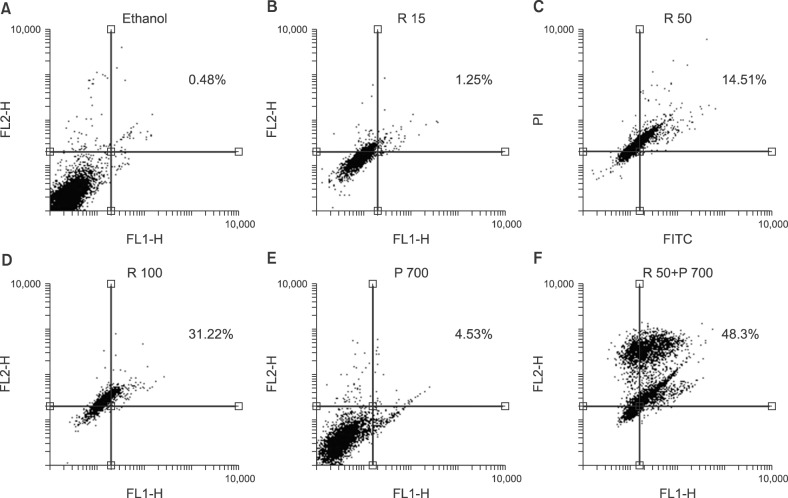
In this study, we investigated the effect of different doses of resveratrol (15, 50 and 100 µM) and prednisolone (700 µM) on BAX (apoptosis promoter) and BCL2 (apoptosis inhibitor) expressions following 24 and 48 hours of treatment on CCRF-CEM cells, using real-time PCR, and on apoptosis induction using flow cytometry.
RESULTS
The results showed a time- and dose-dependent increase in BAX expression and a decrease in BCL2 expression. Apoptosis was induced in CCRF-CEM cells treated with resveratrol and prednisolone for 24 and 48 hours. Combined resveratrol and prednisolone treatment showed synergistic effects on the overexpression of BAX and the downregulation of BCL2. The drug combination had a greater influence on apoptosis induction compared with either drug administered alone after 48 hours of treatment.
CONCLUSION
The results of this study suggested that resveratrol exhibited a remarkable efficacy to improve the influence of glucocorticoids drugs, especially prednisolone, to induce apoptosis in the CCRF-CEM cell line.
Keyword
MeSH Terms
Figure
Reference
-
1. Faderl S, O'Brien S, Pui CH, et al. Adult acute lymphoblastic leukemia: concepts and strategies. Cancer. 2010; 116:1165–1176. PMID: 20101737.2. Tafuri A, Milella M, Iacovelli S, et al. Aberrant proliferative and apoptotic pathways in acute lymphoblastic leukemia (ALL): Molecular therapies to overcome chemo-resistance. In : Stefan Faderl, editor. Novel aspects in acute lymphoblastic leukemia. Rijeka, Croatia: InTech;2011. p. 183–210.3. Dördelmann M, Reiter A, Borkhardt A, et al. Prednisone response is the strongest predictor of treatment outcome in infant acute lymphoblastic leukemia. Blood. 1999; 94:1209–1217. PMID: 10438708.4. Klumper E, Pieters R, Veerman AJ, et al. In vitro cellular drug resistance in children with relapsed/refractory acute lymphoblastic leukemia. Blood. 1995; 86:3861–3868. PMID: 7579354.
Article5. Kaspers GJ, Wijnands JJ, Hartmann R, et al. Immunophenotypic cell lineage and in vitro cellular drug resistance in childhood relapsed acute lymphoblastic leukaemia. Eur J Cancer. 2005; 41:1300–1303. PMID: 15869873.
Article6. Beesley AH, Firth MJ, Ford J, et al. Glucocorticoid resistance in T-lineage acute lymphoblastic leukaemia is associated with a proliferative metabolism. Br J Cancer. 2009; 100:1926–1936. PMID: 19436302.
Article8. Hogarth LA, Hall AG. Increased BAX expression is associated with an increased risk of relapse in childhood acute lymphocytic leukemia. Blood. 1999; 93:2671–2678. PMID: 10194447.
Article9. Campana D, Coustan-Smith E, Manabe A, et al. Prolonged survival of B-lineage acute lymphoblastic leukemia cells is accompanied by overexpression of bcl-2 protein. Blood. 1993; 81:1025–1031. PMID: 8427984.
Article10. Coustan-Smith E, Kitanaka A, Pui CH, et al. Clinical relevance of BCL-2 overexpression in childhood acute lymphoblastic leukemia. Blood. 1996; 87:1140–1146. PMID: 8562940.
Article11. Signorelli P, Ghidoni R. Resveratrol as an anticancer nutrient: molecular basis, open questions and promises. J Nutr Biochem. 2005; 16:449–466. PMID: 16043028.
Article12. Lin MT, Yen ML, Lin CY, Kuo ML. Inhibition of vascular endothelial growth factor-induced angiogenesis by resveratrol through interruption of Src-dependent vascular endothelial cadherin tyrosine phosphorylation. Mol Pharmacol. 2003; 64:1029–1036. PMID: 14573751.
Article13. Seve M, Chimienti F, Devergnas S, et al. Resveratrol enhances UVA-induced DNA damage in HaCaT human keratinocytes. Med Chem. 2005; 1:629–633. PMID: 16787346.
Article14. Cullberg K, Foldager C, Lind M, Richelsen B, Pedersen S. Inhibitory effects of resveratrol on hypoxia-induced inflammation in 3T3-L1 adipocytes and macrophages. J Funct Foods. 2014; 7:171–179.
Article15. Fan GH, Wang ZM, Yang X, et al. Resveratrol inhibits oesophageal adenocarcinoma cell proliferation via AMP-activated protein kinase signaling. Asian Pac J Cancer Prev. 2014; 15:677–682. PMID: 24568477.
Article16. Lephart ED, Sommerfeldt JM, Andrus MB. Resveratrol: influences on gene expression in human skin. J Funct Foods. 2014; 10:377–384.
Article17. Gupta SC, Kannappan R, Reuter S, Kim JH, Aggarwal BB. Chemosensitization of tumors by resveratrol. Ann N Y Acad Sci. 2011; 1215:150–160. PMID: 21261654.
Article18. Dewson G, Kluck RM. Bcl-2 family-regulated apoptosis in health and disease. Cell Health Cytoskelet. 2010; 2:9–22.19. Wojcik I, Szybka M, Golanska E, et al. Abnormalities of the P53, MDM2, BCL2 and BAX genes in acute leukemias. Neoplasma. 2004; 52:318–324.20. Azimi A, Hagh MF, Talebi M, et al. Time-and concentration-dependent effects of resveratrol on miR 15a and miR16-1 expression and apoptosis in the CCRF-CEM acute lymphoblastic leukemia cell line. Asian Pac J Cancer Prev. 2015; 16:6463–6468. PMID: 26434860.21. Tzifi F, Economopoulou C, Gourgiotis D, Ardavanis A, Papageorgiou S, Scorilas A. The role of BCL2 family of apoptosis regulator proteins in acute and chronic leukemias. Adv Hematol. 2012; 2012:524308. PMID: 21941553.
Article22. Winick NJ, Carroll WL, Hunger SP. Childhood leukemia-new advances and challenges. N Engl J Med. 2004; 351:601–603. PMID: 15295054.23. Yin HT, Tian QZ, Guan L, Zhou Y, Huang XE, Zhang H. In vitro and in vivo evaluation of the antitumor efficiency of resveratrol against lung cancer. Asian Pac J Cancer Prev. 2013; 14:1703–1706. PMID: 23679260.
Article24. Jazirehi AR, Bonavida B. Resveratrol modifies the expression of apoptotic regulatory proteins and sensitizes non-Hodgkin's lymphoma and multiple myeloma cell lines to paclitaxel-induced apoptosis. Mol Cancer Ther. 2004; 3:71–84. PMID: 14749477.25. Fulda S, Debatin KM. Sensitization for anticancer drug-induced apoptosis by the chemopreventive agent resveratrol. Oncogene. 2004; 23:6702–6711. PMID: 15273734.
Article26. Bhardwaj A, Sethi G, Vadhan-Raj S, et al. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood. 2007; 109:2293–2302. PMID: 17164350.27. Fouad MA, Agha AM, Merzabani MM, Shouman SA. Resveratrol inhibits proliferation, angiogenesis and induces apoptosis in colon cancer cells: calorie restriction is the force to the cytotoxicity. Hum Exp Toxicol. 2013; 32:1067–1080. PMID: 23536519.28. Dörrie J, Gerauer H, Wachter Y, Zunino SJ. Resveratrol induces extensive apoptosis by depolarizing mitochondrial membranes and activating caspase-9 in acute lymphoblastic leukemia cells. Cancer Res. 2001; 61:4731–4739. PMID: 11406544.29. Estrov Z, Shishodia S, Faderl S, et al. Resveratrol blocks interleukin-1beta-induced activation of the nuclear transcription factor NF-kappaB, inhibits proliferation, causes S-phase arrest, and induces apoptosis of acute myeloid leukemia cells. Blood. 2003; 102:987–995. PMID: 12689943.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Significance of Apoptotic Cell Death after gamma-Irradiation
- Inhibitory effect of temozolomide on apoptosis induction of cinnamaldehyde in human glioblastoma multiforme T98G cell line
- The Cardioprotective Effects of Resveratrol via Anti-Apoptosis in Hypoxic Injury of Myocardial Cells
- Real-time PCR analysis of the apoptosis related genes in ATRA treated APL t(15;17) patients
- Effect on Cell Growth, c-myc mRNA Expression and Telomerase Activity by Transforming Growth Factor-beta1 in Malignant Lymphoma and Leukemia Cell Line