Diabetes Metab J.
2018 Jun;42(3):233-243. 10.4093/dmj.2017.0084.
Inhibition of Serotonin Synthesis Induces Negative Hepatic Lipid Balance
- Affiliations
-
- 1Graduate School of Medical Science and Engineering, Korea Advanced Institute of Science and Technology, Daejeon, Korea. 49park@cku.ac.kr, hailkim@kaist.edu
- 2Department of Biochemistry, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 3Biomedical Science and Engineering Interdisciplinary Program, Korea Advanced Institute of Science and Technology, Daejeon, Korea.
- 4Department of Biochemistry, Catholic Kwandong University College of Medicine, Gangneung, Korea.
- KMID: 2413986
- DOI: http://doi.org/10.4093/dmj.2017.0084
Abstract
- BACKGROUND
Hepatic steatosis is caused by metabolic stress associated with a positive lipid balance, such as insulin resistance and obesity. Previously we have shown the anti-obesity effects of inhibiting serotonin synthesis, which eventually improved insulin sensitivity and hepatic steatosis. However, it is not clear whether serotonin has direct effect on hepatic lipid accumulation. Here, we showed the possibility of direct action of serotonin on hepatic steatosis.
METHODS
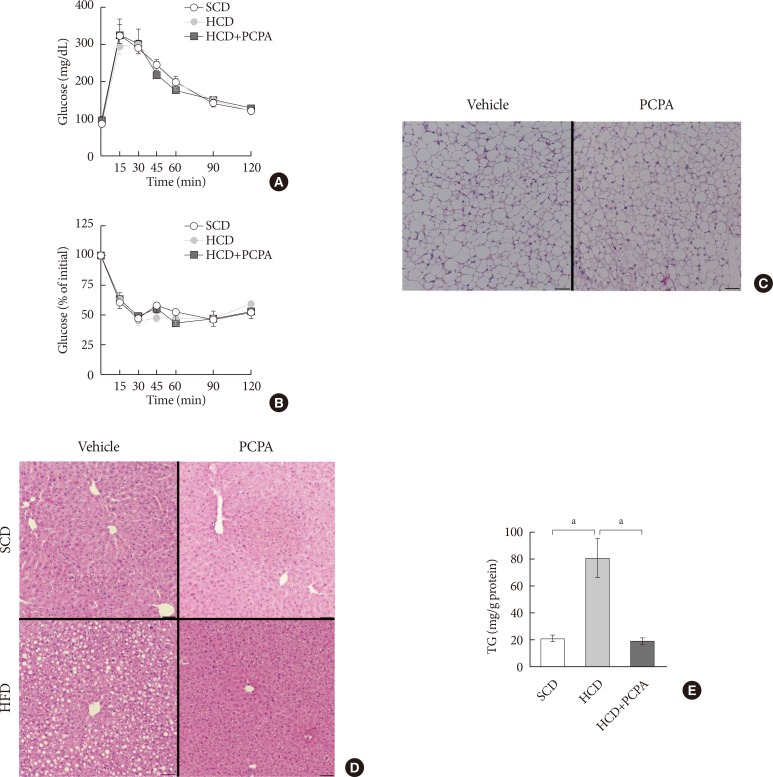
Mice were treated with para-chlorophenylalanine (PCPA) or LP-533401 to inhibit serotonin synthesis and fed with high fat diet (HFD) or high carbohydrate diet (HCD) to induce hepatic steatosis. Hepatic triglyceride content and gene expression profiles were analyzed.
RESULTS
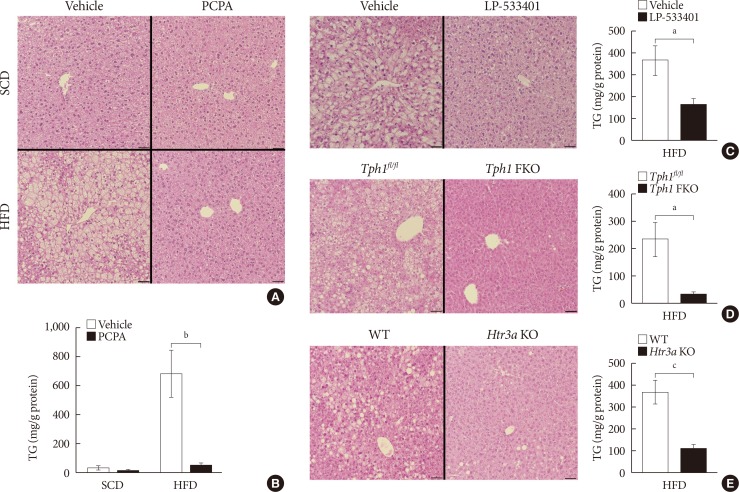
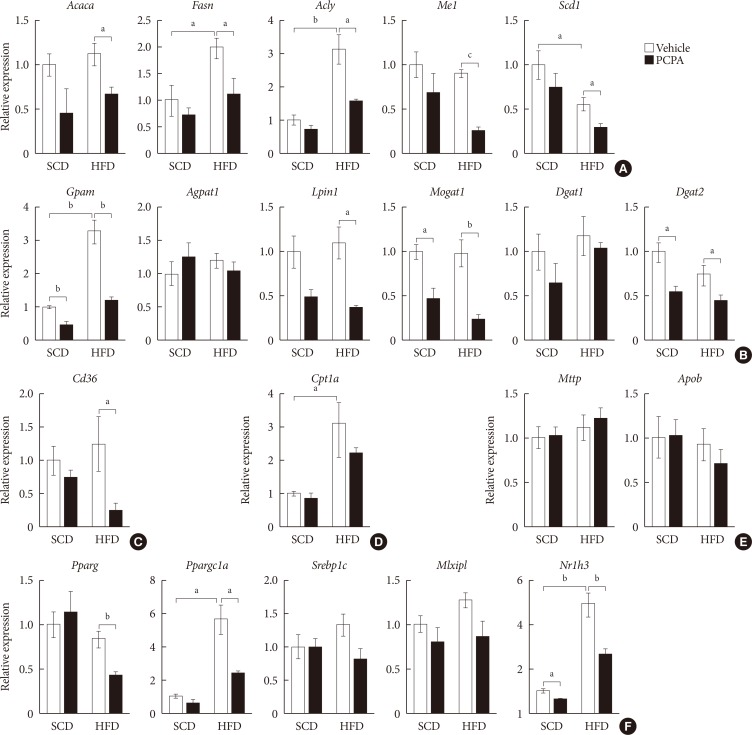
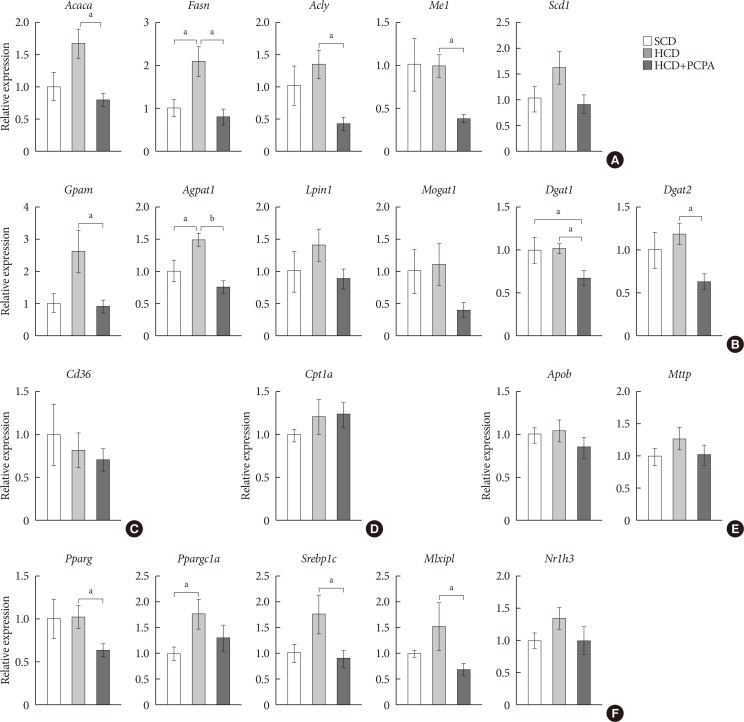
Pharmacological and genetic inhibition of serotonin synthesis reduced HFD-induced hepatic lipid accumulation. Furthermore, short-term PCPA treatment prevented HCD-induced hepatic steatosis without affecting glucose tolerance and browning of subcutaneous adipose tissue. Gene expression analysis revealed that the expressions of genes involved in de novo lipogenesis and triacylglycerol synthesis were downregulated by short-term PCPA treatment as well as long-term PCPA treatment.
CONCLUSION
Short-term inhibition of serotonin synthesis prevented hepatic lipid accumulation without affecting systemic insulin sensitivity and energy expenditure, suggesting the direct steatogenic effect of serotonin in liver.
Keyword
MeSH Terms
Figure
Reference
-
1. Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006; 43(2 Suppl 1):S99–S112. PMID: 16447287.
Article2. Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol. 2012; 56:1384–1391. PMID: 22326465.
Article3. Adams LA, Waters OR, Knuiman MW, Elliott RR, Olynyk JK. NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: an eleven-year follow-up study. Am J Gastroenterol. 2009; 104:861–867. PMID: 19293782.
Article4. Kawano Y, Cohen DE. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J Gastroenterol. 2013; 48:434–441. PMID: 23397118.
Article5. Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013; 93:359–404. PMID: 23303913.
Article6. Galgani J, Ravussin E. Energy metabolism, fuel selection and body weight regulation. Int J Obes (Lond). 2008; 32(Suppl 7):S109–S119. PMID: 19136979.
Article7. Golabi P, Bush H, Younossi ZM. Treatment strategies for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Clin Liver Dis. 2017; 21:739–753. PMID: 28987260.
Article8. Samuel VT, Shulman GI. Nonalcoholic fatty liver disease as a nexus of metabolic and hepatic diseases. Cell Metab. 2018; 27:22–41. PMID: 28867301.
Article9. Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009; 60:355–366. PMID: 19630576.
Article10. Watanabe H, Rose MT, Aso H. Role of peripheral serotonin in glucose and lipid metabolism. Curr Opin Lipidol. 2011; 22:186–191. PMID: 21494145.
Article11. Kim HJ, Kim JH, Noh S, Hur HJ, Sung MJ, Hwang JT, Park JH, Yang HJ, Kim MS, Kwon DY, Yoon SH. Metabolomic analysis of livers and serum from high-fat diet induced obese mice. J Proteome Res. 2011; 10:722–731. PMID: 21047143.
Article12. Chen X, Margolis KJ, Gershon MD, Schwartz GJ, Sze JY. Reduced serotonin reuptake transporter (SERT) function causes insulin resistance and hepatic steatosis independent of food intake. PLoS One. 2012; 7:e32511. PMID: 22412882.
Article13. Uceyler N, Schutt M, Palm F, Vogel C, Meier M, Schmitt A, Lesch KP, Mossner R, Sommer C. Lack of the serotonin transporter in mice reduces locomotor activity and leads to gender-dependent late onset obesity. Int J Obes (Lond). 2010; 34:701–711. PMID: 20084070.14. Oh CM, Namkung J, Go Y, Shong KE, Kim K, Kim H, Park BY, Lee HW, Jeon YH, Song J, Shong M, Yadav VK, Karsenty G, Kajimura S, Lee IK, Park S, Kim H. Regulation of systemic energy homeostasis by serotonin in adipose tissues. Nat Commun. 2015; 6:6794. PMID: 25864946.
Article15. Crane JD, Palanivel R, Mottillo EP, Bujak AL, Wang H, Ford RJ, Collins A, Blumer RM, Fullerton MD, Yabut JM, Kim JJ, Ghia JE, Hamza SM, Morrison KM, Schertzer JD, Dyck JR, Khan WI, Steinberg GR. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat Med. 2015; 21:166–172. PMID: 25485911.
Article16. Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schutz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, Mann JJ, Hen R, Ducy P, Karsenty G. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008; 135:825–837. PMID: 19041748.
Article17. Eguchi J, Wang X, Yu S, Kershaw EE, Chiu PC, Dushay J, Estall JL, Klein U, Maratos-Flier E, Rosen ED. Transcriptional control of adipose lipid handling by IRF4. Cell Metab. 2011; 13:249–259. PMID: 21356515.
Article18. Fischer AH, Jacobson KA, Rose J, Zeller R. Cutting sections of paraffin-embedded tissues. CSH Protoc. 2008; 2008:pdb.prot4987. PMID: 21356830.
Article19. Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of issue and cell sections. CSH Protoc. 2008; 2008:pdb.prot4986. PMID: 21356829.20. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-delta delta C (T)) Method. Methods. 2001; 25:402–408. PMID: 11846609.21. Breisch ST, Zemlan FP, Hoebel BG. Hyperphagia and obesity following serotonin depletion by intraventricular p-chlorophenylalanine. Science. 1976; 192:382–385. PMID: 130678.22. Liu Q, Yang Q, Sun W, Vogel P, Heydorn W, Yu XQ, Hu Z, Yu W, Jonas B, Pineda R, Calderon-Gay V, Germann M, O'Neill E, Brommage R, Cullinan E, Platt K, Wilson A, Powell D, Sands A, Zambrowicz B, Shi ZC. Discovery and characterization of novel tryptophan hydroxylase inhibitors that selectively inhibit serotonin synthesis in the gastrointestinal tract. J Pharmacol Exp Ther. 2008; 325:47–55. PMID: 18192499.
Article23. Marchesini G, Petta S, Dalle Grave R. Diet, weight loss, and liver health in nonalcoholic fatty liver disease: pathophysiology, evidence, and practice. Hepatology. 2016; 63:2032–2043. PMID: 26663351.
Article24. Sumara G, Sumara O, Kim JK, Karsenty G. Gut-derived serotonin is a multifunctional determinant to fasting adaptation. Cell Metab. 2012; 16:588–600. PMID: 23085101.
Article25. Koe BK, Weissman A. P-chlorophenylalanine: a specific depletor of brain serotonin. J Pharmacol Exp Ther. 1966; 154:499–516. PMID: 5297133.26. Sanders-Bush E, Sulser F. P-chloroamphetamine: in vivo investigations on the mechanism of action of the selective depletion of cerebral serotonin. J Pharmacol Exp Ther. 1970; 175:419–426. PMID: 5312363.27. Engelman K, Lovenberg W, Sjoerdsma A. Inhibition of serotonin synthesis by para-chlorophenylalanine in patients with the carcinoid syndrome. N Engl J Med. 1967; 277:1103–1108. PMID: 6054996.
Article28. Nonogaki K, Strack AM, Dallman MF, Tecott LH. Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nat Med. 1998; 4:1152–1156. PMID: 9771748.
Article29. Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004; 114:147–152. PMID: 15254578.
Article30. Postic C, Dentin R, Denechaud PD, Girard J. ChREBP, a transcriptional regulator of glucose and lipid metabolism. Annu Rev Nutr. 2007; 27:179–192. PMID: 17428181.
Article31. Pettinelli P, Videla LA. Up-regulation of PPAR-gamma mRNA expression in the liver of obese patients: an additional reinforcing lipogenic mechanism to SREBP-1c induction. J Clin Endocrinol Metab. 2011; 96:1424–1430. PMID: 21325464.32. Rozenblit-Susan S, Chapnik N, Froy O. Metabolic effect of fluvoxamine in mouse peripheral tissues. Mol Cell Endocrinol. 2016; 424:12–22. PMID: 26797245.
Article33. Osawa Y, Kanamori H, Seki E, Hoshi M, Ohtaki H, Yasuda Y, Ito H, Suetsugu A, Nagaki M, Moriwaki H, Saito K, Seishima M. L-tryptophan-mediated enhancement of susceptibility to nonalcoholic fatty liver disease is dependent on the mammalian target of rapamycin. J Biol Chem. 2011; 286:34800–34808. PMID: 21841000.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sour cherry ameliorates hepatic lipid synthesis in high-fat diet-induced obese mice via activation of adenosine monophosphate-activated protein kinase signaling
- Serotonin Regulates De Novo Lipogenesis in Adipose Tissues through Serotonin Receptor 2A
- Hepatic Expression of the Serine Palmitoyltransferase Subunit Sptlc2 Reduces Lipid Droplets in the Liver by Activating VLDL Secretion
- Lipid A as a Drug Target and Therapeutic Molecule
- Intravital Two-photon Imaging of Dynamic Alteration of Hepatic Lipid Droplets in Fasted and Refed State