J Breast Cancer.
2018 Jun;21(2):182-189. 10.4048/jbc.2018.21.2.182.
Assessment of Quality of Life and Safety in Postmenopausal Breast Cancer Patients Receiving Letrozole as an Early Adjuvant Treatment
- Affiliations
-
- 1Department of Surgery, Ajou University School of Medicine, Suwon, Korea. hanse@aumc.ac.kr
- 2Department of Surgery, Yeungnam University Hospital, Daegu, Korea.
- 3Department of Biostatistics, Korea University College of Medicine, Seoul, Korea.
- 4Department of Surgery, Korea Institute of Radiological and Medical Science, Seoul, Korea.
- 5Department of Surgery, Samsung Medical Center, Seoul, Korea.
- 6Department of Surgery, Yonsei University College of Medicine, Seoul, Korea.
- 7Department of Surgery, Pusan National University School of Medicine, Busan, Korea.
- 8Department of Surgery, Cheil General Hospital & Women's Healthcare Center, Dankook University College of Medicine, Seoul, Korea.
- 9Department of Surgery, Gachon University Gil Hospital, Incheon, Korea.
- 10Department of Surgery, Chonnam National University Hwasun Hospital, Hwasun, Korea.
- 11Department of Surgery, Chungnam National University Hospital, Daejeon, Korea.
- 12Department of Surgery, Dong-A University Hospital, Busan, Korea.
- 13Department of Surgery, Hallym University College of Medicine, Anyang, Korea.
- 14Breast Cancer Center, Ewha Womans University Hospital, Seoul, Korea.
- 15Department of Surgery, Chonbuk National University Medical School, Jeonju, Korea.
- 16Department of Surgery, Soonchunhyang University Bucheon Hospital, Bucheon, Korea.
- 17Department of Surgery, Soonchunhyang University Cheonan Hospital, Cheonan, Korea.
- 18Department of Surgery, Kyungpook National University Medical Center, Daegu, Korea.
- 19Department of Surgery, Kyung Hee University Hospital at Gangdong, Seoul, Korea.
- 20Department of Surgery, Wonkwang University Hospital, Iksan, Korea.
- 21Department of Surgery, Daegu Catholic University Hospital, Daegu, Korea.
- 22Department of Surgery, Yonsei University Gangnam Severance Hospital, Seoul, Korea.
- 23Department of Surgery, CHA Gangnam Medical Center, Seoul, Korea.
- 24Department of Surgery, Daerim St. Mary's Hospital, Seoul, Korea.
- 25Department of Surgery, Dongguk University Ilsan Hospital, Goyang, Korea.
- 26Department of Surgery, Keimyung University School of Medicine, Daegu, Korea.
- 27Department of Surgery, Inje University Sanggye Paik Hospital, Seoul, Korea.
- 28Department of Surgery, Kangbuk Samsung Hospital, Seoul, Korea.
- 29Department of Surgery, Bundang Jesaeng Hospital, Seongnam, Korea.
- KMID: 2413937
- DOI: http://doi.org/10.4048/jbc.2018.21.2.182
Abstract
- PURPOSE
There are few reports from Asian countries about the long-term results of aromatase inhibitor adjuvant treatment for breast cancer. This observational study aimed to evaluate the long-term effects of letrozole in postmenopausal Korean women with operable breast cancer.
METHODS
Self-reported quality of life (QoL) scores were serially assessed for 3 years during adjuvant letrozole treatment using the Korean version of the Functional Assessment of Cancer Therapy-Breast questionnaires (version 3). Changes in bone mineral density (BMD) and serum cholesterol levels were also examined.
RESULTS
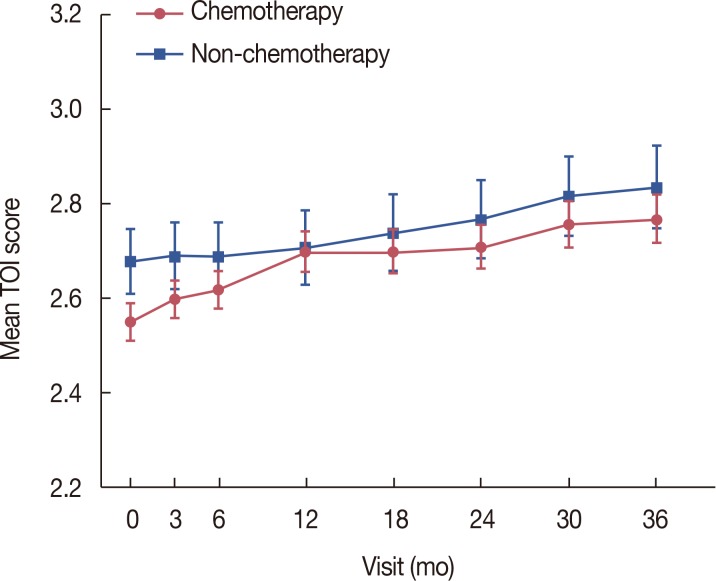
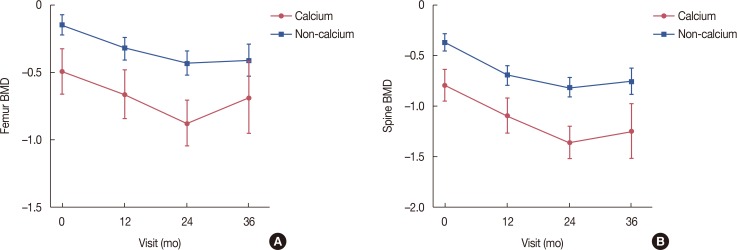
All 897 patients received the documented informed consent form and completed a baseline questionnaire before treatment. Adjuvant chemotherapy was administered to 684 (76.3%) subjects, and 410 (45.7%) and 396 (44.1%) patients had stage I and II breast cancer, respectively. Each patient completed questionnaires at 3, 6, 12, 18, 24, 30, and 36 months after enrollment. Of 897 patients, 749 (83.5%) completed the study. The dropout rate was 16.5%. The serial trial outcome index, the sum of the physical and functional well-being subscales, increased gradually and significantly from baseline during letrozole treatment (p<0.001). The mean serum cholesterol level increased significantly from 199 to 205 after 36 months (p=0.042). The mean BMD significantly decreased from −0.39 at baseline to −0.87 after 36 months (p<0.001).
CONCLUSION
QoL gradually improved during letrozole treatment. BMD and serum cholesterol level changes were similar to those in Western countries, indicating that adjuvant letrozole treatment is well tolerated in Korean women, with minimal ethnic variation.
Keyword
MeSH Terms
Figure
Reference
-
1. Park EH, Min SY, Kim Z, Yoon CS, Jung KW, Nam SJ, et al. Basic facts of breast cancer in Korea in 2014: the 10-year overall survival progress. J Breast Cancer. 2017; 20:1–11. PMID: 28382089.
Article2. Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013; 24:2206–2223. PMID: 23917950.3. Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005; 365:60–62. PMID: 15639680.4. Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004; 350:1081–1092. PMID: 15014181.
Article5. BIG 1-98 Collaborative Group. Mouridsen H, Giobbie-Hurder A, Goldhirsch A, Thürlimann B, Paridaens R, et al. Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med. 2009; 361:766–776. PMID: 19692688.
Article6. Arimidex, Tamoxifen, Alone or in Combination (ATAC) Trialists' Group. Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008; 9:45–53. PMID: 18083636.7. Geisler J, Haynes B, Anker G, Dowsett M, Lønning PE. Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, cross-over study. J Clin Oncol. 2002; 20:751–757. PMID: 11821457.
Article8. Ohsumi S, Shimozuma K, Ohashi Y, Shinji M, Hozumi Y, Mukai H, et al. Health-related quality of life and psychological distress of breast cancer patients after surgery during a phase III randomized trial comparing continuation of tamoxifen with switching to anastrozole after adjuvant tamoxifen for 1-4 years: N-SAS BC 03. Breast Cancer Res Treat. 2011; 127:143–152. PMID: 21347648.9. Hozumi Y, Suemasu K, Takei H, Aihara T, Takehara M, Saito T, et al. The effect of exemestane, anastrozole, and tamoxifen on lipid profiles in Japanese postmenopausal early breast cancer patients: final results of National Surgical Adjuvant Study BC 04, the TEAM Japan sub-study. Ann Oncol. 2011; 22:1777–1782. PMID: 21285133.
Article10. Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol. 1997; 15:974–986. PMID: 9060536.
Article11. Cella D, Hahn EA, Dineen K. Meaningful change in cancer-specific quality of life scores: differences between improvement and worsening. Qual Life Res. 2002; 11:207–221. PMID: 12074259.12. Fallowfield LJ, Leaity SK, Howell A, Benson S, Cella D. Assessment of quality of life in women undergoing hormonal therapy for breast cancer: validation of an endocrine symptom subscale for the FACT-B. Breast Cancer Res Treat. 1999; 55:189–199. PMID: 10481946.
Article13. Fallowfield L, Cella D, Cuzick J, Francis S, Locker G, Howell A. Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) Adjuvant Breast Cancer Trial. J Clin Oncol. 2004; 22:4261–4271. PMID: 15514369.
Article14. Whelan TJ, Goss PE, Ingle JN, Pater JL, Tu D, Pritchard K, et al. Assessment of quality of life in MA.17: a randomized, placebo-controlled trial of letrozole after 5 years of tamoxifen in postmenopausal women. J Clin Oncol. 2005; 23:6931–6940. PMID: 16157934.
Article15. Mincey BA, Duh MS, Thomas SK, Moyneur E, Marynchencko M, Boyce SP, et al. Risk of cancer treatment-associated bone loss and fractures among women with breast cancer receiving aromatase inhibitors. Clin Breast Cancer. 2006; 7:127–132. PMID: 16800971.
Article16. Muss HB, Tu D, Ingle JN, Martino S, Robert NJ, Pater JL, et al. Efficacy, toxicity, and quality of life in older women with early-stage breast cancer treated with letrozole or placebo after 5 years of tamoxifen: NCIC CTG intergroup trial MA.17. J Clin Oncol. 2008; 26:1956–1964. PMID: 18332474.
Article17. Muss HB. Adjuvant treatment of elderly breast cancer patients. Breast. 2007; 16(Suppl 2):S159–S165. PMID: 17728133.
Article18. Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003; 21:602–606. PMID: 12586795.
Article19. Cuzick J, Sestak I, Cella D, Fallowfield L. ATAC Trialists Group. Treatment-emergent endocrine symptoms and the risk of breast cancer recurrence: a retrospective analysis of the ATAC trial. Lancet Oncol. 2008; 9:1143–1148. PMID: 18976959.
Article20. Zaman K, Thürlimann B, Huober J, Schönenberger A, Pagani O, Lüthi J, et al. Bone mineral density in breast cancer patients treated with adjuvant letrozole, tamoxifen, or sequences of letrozole and tamoxifen in the BIG 1-98 study (SAKK 21/07). Ann Oncol. 2012; 23:1474–1481. PMID: 22003243.
Article21. Perez EA, Josse RG, Pritchard KI, Ingle JN, Martino S, Findlay BP, et al. Effect of letrozole versus placebo on bone mineral density in women with primary breast cancer completing 5 or more years of adjuvant tamoxifen: a companion study to NCIC CTG MA.17. J Clin Oncol. 2006; 24:3629–3635. PMID: 16822845.
Article22. Brufsky A, Harker WG, Beck JT, Carroll R, Tan-Chiu E, Seidler C, et al. Zoledronic acid inhibits adjuvant letrozole-induced bone loss in postmenopausal women with early breast cancer. J Clin Oncol. 2007; 25:829–836. PMID: 17159193.
Article23. Sestak I, Singh S, Cuzick J, Blake GM, Patel R, Gossiel F, et al. Changes in bone mineral density at 3 years in postmenopausal women receiving anastrozole and risedronate in the IBIS-II bone substudy: an international, double-blind, randomised, placebo-controlled trial. Lancet Oncol. 2014; 15:1460–1468. PMID: 25456365.
Article24. Wasan KM, Goss PE, Pritchard PH, Shepherd L, Palmer MJ, Liu S, et al. The influence of letrozole on serum lipid concentrations in postmenopausal women with primary breast cancer who have completed 5 years of adjuvant tamoxifen (NCIC CTG MA.17L). Ann Oncol. 2005; 16:707–715. PMID: 15817595.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes of Symptom Distress and Quality of Life in Breast Cancer Patients Receiving Adjuvant Therapy
- Cost-Effectiveness Analysis of Adjuvant Hormonal Treatments for Women with Postmenopausal Hormone-Receptor Positive Early Breast Cancer in the Korean Context
- An Effect of Letrozole on Gastric Cancer?
- Hormonal Changes during Extended Letrozole Treatment after Completion of 5 Years of Tamoxifen in Premenopausal Patients with Breast Cancer who Became Postmenopausal
- Survival Benefit of Zoledronic Acid in Postmenopausal Breast Cancer Patients Receiving Aromatase Inhibitors