Transl Clin Pharmacol.
2016 Dec;24(4):175-182. 10.12793/tcp.2016.24.4.175.
Pharmacokinetics and safety profiles of tadalafil/tamsulosin HCl fixed-dose combination capsule under fasted and fed condition in healthy volunteers
- Affiliations
-
- 1Department of Pharmaceutical Medicine and Regulatory Sciences, College of Medicine and Pharmacy, Yonsei University, Incheon 21983, Korea. minspark@yuhs.ac
- 2Department of Clinical Pharmacology and Clinical Trials Center, Severance Hospital, College of Medicine, Yonsei University, Seoul 03722, Korea.
- 3Department of Pharmacy, College of Pharmacy, Yonsei University, Incheon 21983, Korea.
- 4Yonsei Institute of Pharmaceutical Sciences, College of Pharmacy, Yonsei University, Incheon 21983, Korea.
- 5Clinical Research Team, Hanmi Pharmaceutical Co.,Ltd., Seoul 05545, Korea.
- 6Department of Pediatrics, College of Medicine, Yonsei University, Seoul 03722, Korea.
- KMID: 2413839
- DOI: http://doi.org/10.12793/tcp.2016.24.4.175
Abstract
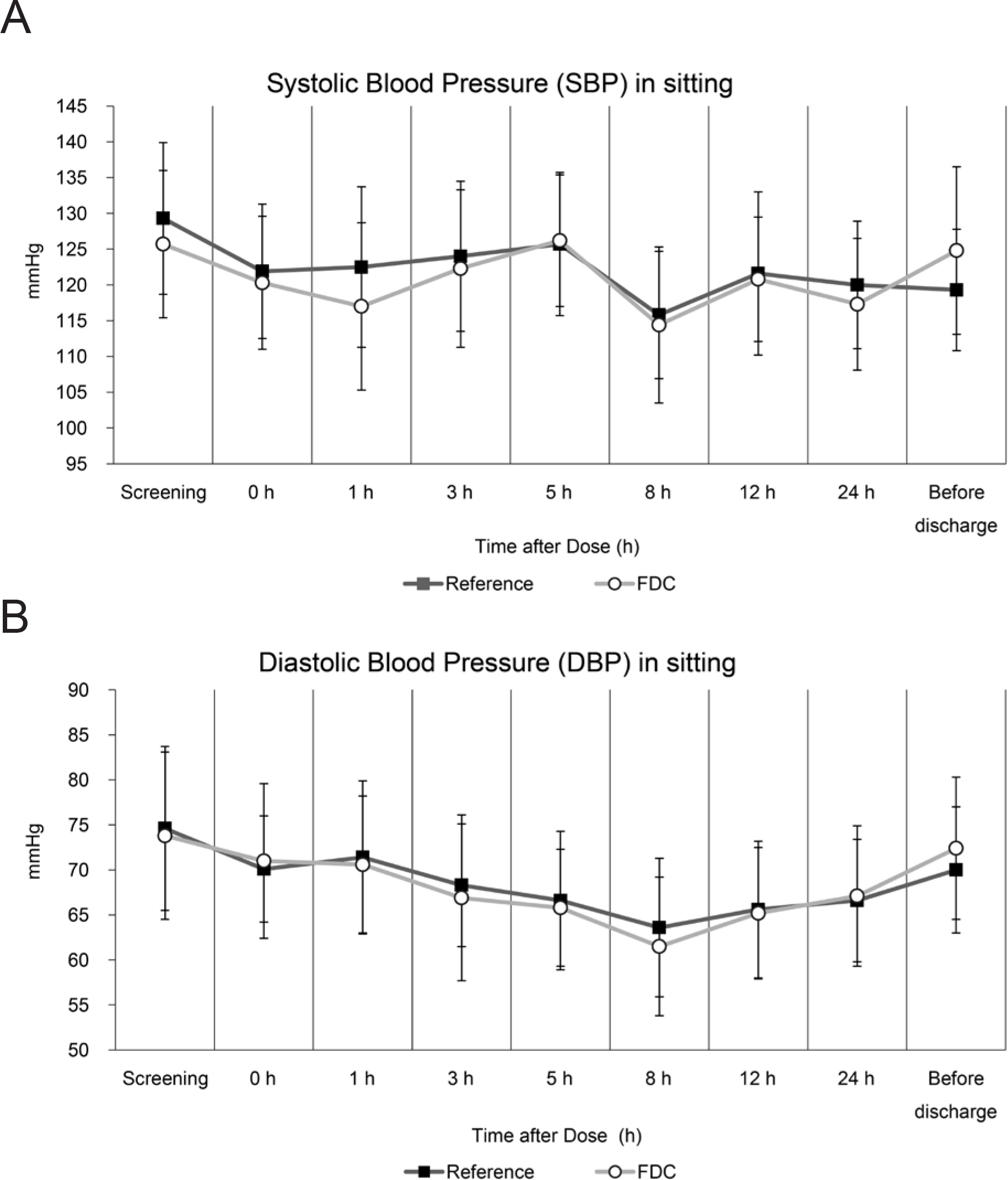
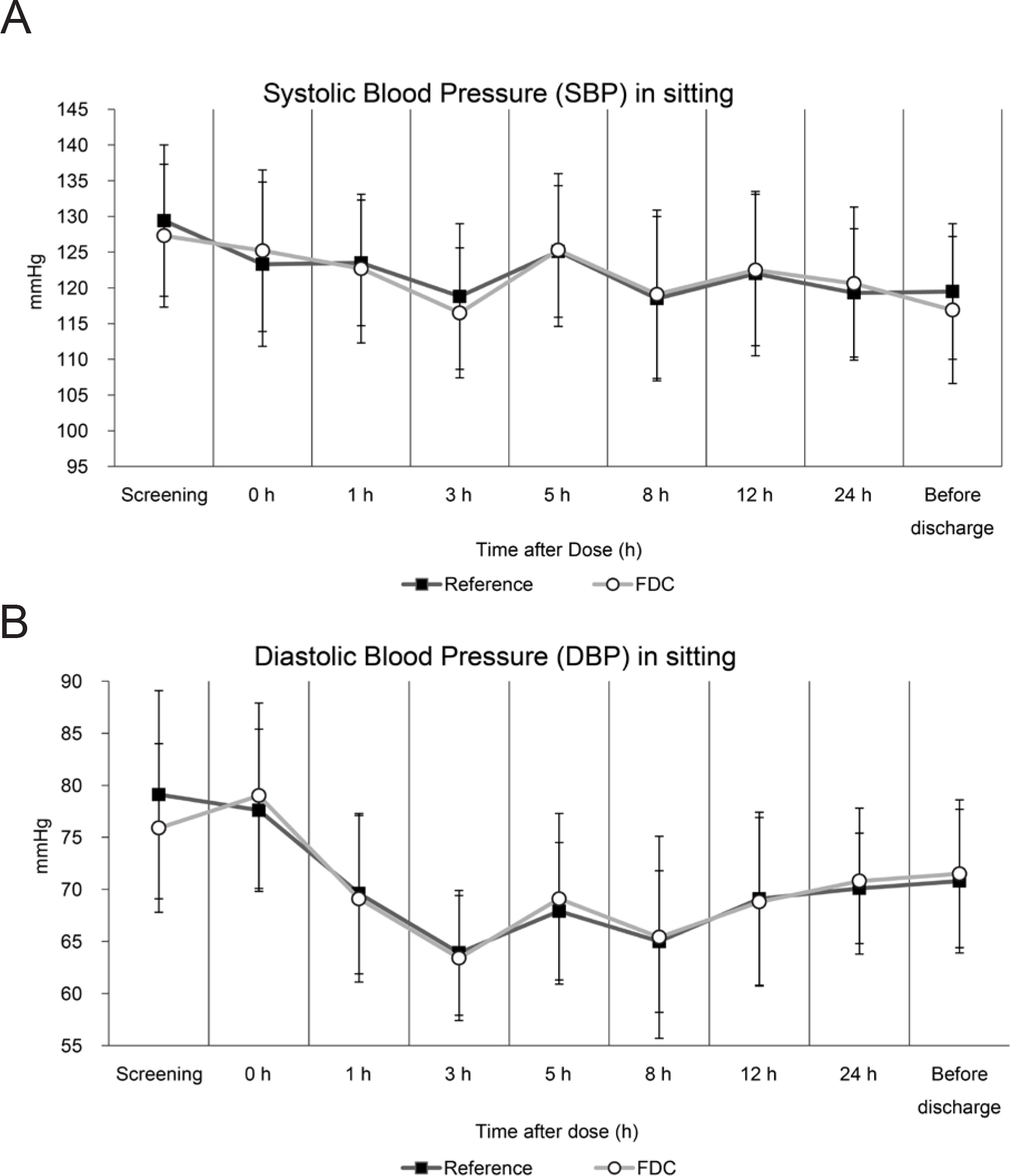
- Co-administration of tadalafil and tamsulosin HCl in patients with benign prostate hyperplasia and erectile dysfunction is increasing in clinical settings. Development of fixed-dose combination (FDC) of tadalafil and tamsulosin HCl could contribute to improving patients' adherence and treatment efficacy. We evaluated the pharmacokinetics and safety profiles of a newly developed fixed-dose combination capsule of tadalafil 5 mg/tamsulosin HCl 0.4 mg in comparison with co-administration of each formulation in healthy volunteers under fasted and fed conditions. Two randomized, open-label, single-dose, two-way, crossover studies were completed in 29 subjects under fasted condition, and 33 subjects under fed condition. Serial blood sample collection for PK analysis was conducted up to 72 hours after dosing, and PK parameters were calculated using non-compartmental analysis. Geometric mean ratios and 90% confidence intervals of the C(max) and AUC(last) were used to evaluate comparative bioavailability. In both fasted and fed condition studies, the bioequivalence was established. The most common adverse drug reactions were orthostatic hypotension and headache with no statistical difference between treatment groups. All subjects with orthostatic hypotension recovered at follow-up test. Although changes in vital signs from baseline were statistically significant, there were no subjects with systolic blood pressure < 90 mmHg and there were no clinically meaningful signs or symptoms associated. FDC of tadalafil and tamsulosin HCl can be an alternative to co-administration of individual drugs for providing better compliance. Changes in blood pressure should be kept in mind when tadalafil and tamsulosin HCl are co-administered in clinical settings.
MeSH Terms
-
Biological Availability
Blood Pressure
Compliance
Cross-Over Studies
Drug-Related Side Effects and Adverse Reactions
Erectile Dysfunction
Follow-Up Studies
Headache
Healthy Volunteers*
Humans
Hyperplasia
Hypotension, Orthostatic
Male
Pharmacokinetics*
Prostate
Tadalafil
Therapeutic Equivalency
Treatment Outcome
Vital Signs
Tadalafil
Figure
Reference
-
1.Auffenberg GB., Helfand BT., McVary KT. Established medical therapy for benign prostatic hyperplasia. Urol Clin North Am. 2009. 36:443–59. v-vi.
Article2.Lee C., Kozlowski JM., Grayhack JT. Intrinsic and extrinsic factors controlling benign prostatic growth. Prostate. 1997. 31:131–138.
Article3.Garraway WM., Collins GN., Lee RJ. High prevalence of benign prostatic hypertrophy in the community. Lancet. 1991. 338:469–471.
Article4.Park HK., Park H., Cho SY., Bae J., Jeong SJ., Hong SK, et al. The prevalence of benign prostatic hyperplasia in elderly men in Korea: A community-based study. Kor J Urol. 2009. 50:843–847.
Article5.Bae WJ., Sohn DW., Kim SD., Kim SJ., Hong SH., Lee JY, et al. The correlation between cardiovascular risk factors and penile hemodynamic parameters in men with erectile dysfunction. Kor J Urol. 2009. 50:689–693.
Article6.Braun M., Wassmer G., Klotz T., Reifenrath B., Mathers M., Engelmann U. Epidemiology of erectile dysfunction: results of the 'Cologne Male Survey'. Int J Impot Res. 2000. 12:305–311.
Article7.McVary KT., Roehrborn CG., Kaminetsky JC., Auerbach SM., Wachs B., Young JM, et al. Tadalafil relieves lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2007. 177:1401–1407.
Article8.Oh SY., Min KS., Choi SH. Effects of prostate volume and lower urinary tract symptoms on erectile function. Kor J Urol. 2007. 48:24–28.
Article9.U. S. Food and Drug Administration. Flomax Capsules, 0.4mg Prescribing Information. Accessed 20 June 2016.http://www.accessdata.fda.gov/drugsatfda_docs/label/2005/020579s016lbl.pdf.10.Montorsi F., Verheyden B., Meuleman E., Jünemann KP., Moncada I., Valiquette L, et al. Long-term safety and tolerability of tadalafil in the treatment of erectile dysfunction. Eur Urol. 2004. 45:339–344. discussion 344-345.
Article11.McMahon CG. Treatment of erectile dysfunction with chronic dosing of tadalafil. Eur Urol. 2006. 50:215–217.
Article12.U. S. Food and Drug Administration. CIALIS Highlights of Prescribing Information. Accessed 20 June 2016.http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021368s20s21lbl.pdf.13.Bechara A., Romano S., Casabé A., Haime S., Dedola P., Hernández C, et al. Comparative efficacy assessment of tamsulosin vs. tamsulosin plus tadalafil in the treatment of LUTS/BPH. Pilot study. J Sex Med. 2008. 5:2170–2178.
Article14.Reges R., Regadas R., Moraes F., Manoel O., Jamacaru F., Vagnaldo F, et al. The association of tamsulosin and daily tadalafil for the treatment of lower urianry tract symptoms is safe and effective? J Urol. 2012. 187:e507.
Article15.Forgue ST., Patterson BE., Bedding AW., Payne CD., Phillips DL., Wrishko RE, et al. Tadalafil pharmacokinetics in healthy subjects. Br J Clin Pharmacol. 2006. 61:280–288.
Article16.World Health Organization. Guidelines for registration of fixed-dose combination medicinal products, in WHO Technical Report Series. 2005.17.Chapple CR. Selective alpha 1-adrenoceptor antagonists in benign prostatic hyperplasia: rationale and clinical experience. Eur Urol. 1996. 29:129–144.18.de Mey C. Cardiovascular effects of alpha-blockers used for the treatment of symptomatic BPH: impact on safety and well-being. Eur Urol. 1998. 34(Suppl 2):18-28; discussion 47.
Article19.Yasukawa K., Swarz H., Ito Y. Review of orthostatic tests on the safety of tamsulosin, a selective alpha1A-adrenergic receptor antagonist, shows lack of orthostatic hypotensive effects. J Int Med Res. 2001. 29:236–251.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of the pharmacokinetics and food effects of a novel formulation tamsulosin 0.4 mg capsule compared with a 0.2 mg capsule in healthy male volunteers
- Pharmacokinetics and pharmacodynamics of a fixed-dose combination of gemigliptin/metformin sustained release 25/500 mg compared to the loose combination in healthy male subjects
- Steady-State Pharmacokinetic Properties of Tamsulosin in Healthy Male Volunteers
- Treatment persistence with a fixed-dose combination of tadalafil (5 mg) and tamsulosin (0.4 mg) and reasons for early discontinuation in patients with benign prostatic hyperplasia and erectile dysfunction
- Fed and fasted bioequivalence assessment of two formulations of extended-release fixed-dose combination dapagliflozin/metformin (10/1,000 mg) tablets in healthy subjects