Transl Clin Pharmacol.
2018 Jun;26(2):79-85. 10.12793/tcp.2018.26.2.79.
Effect of plasma membrane monoamine transporter genetic variants on pharmacokinetics of metformin in humans
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Hospital, Seoul 03080, Republic of Korea.
- 2Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Bundang Hospital, Seongnam 13620, Republic of Korea. jychung@snubh.org
- KMID: 2413831
- DOI: http://doi.org/10.12793/tcp.2018.26.2.79
Abstract
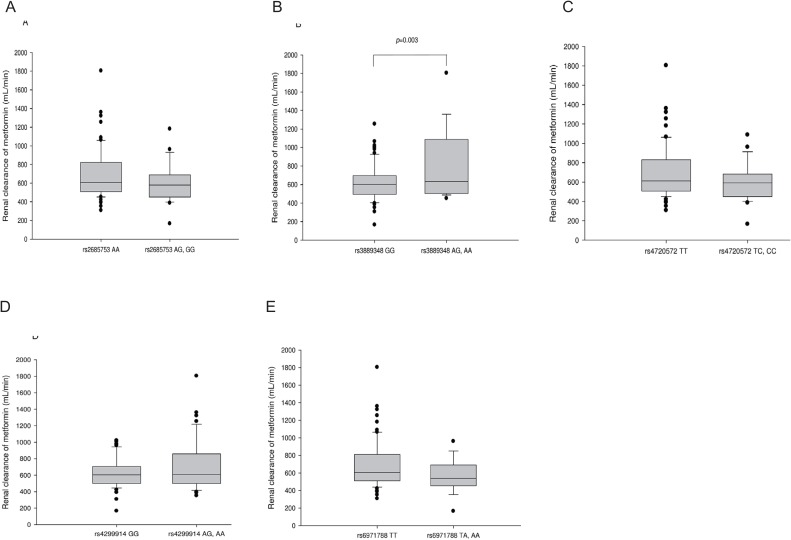
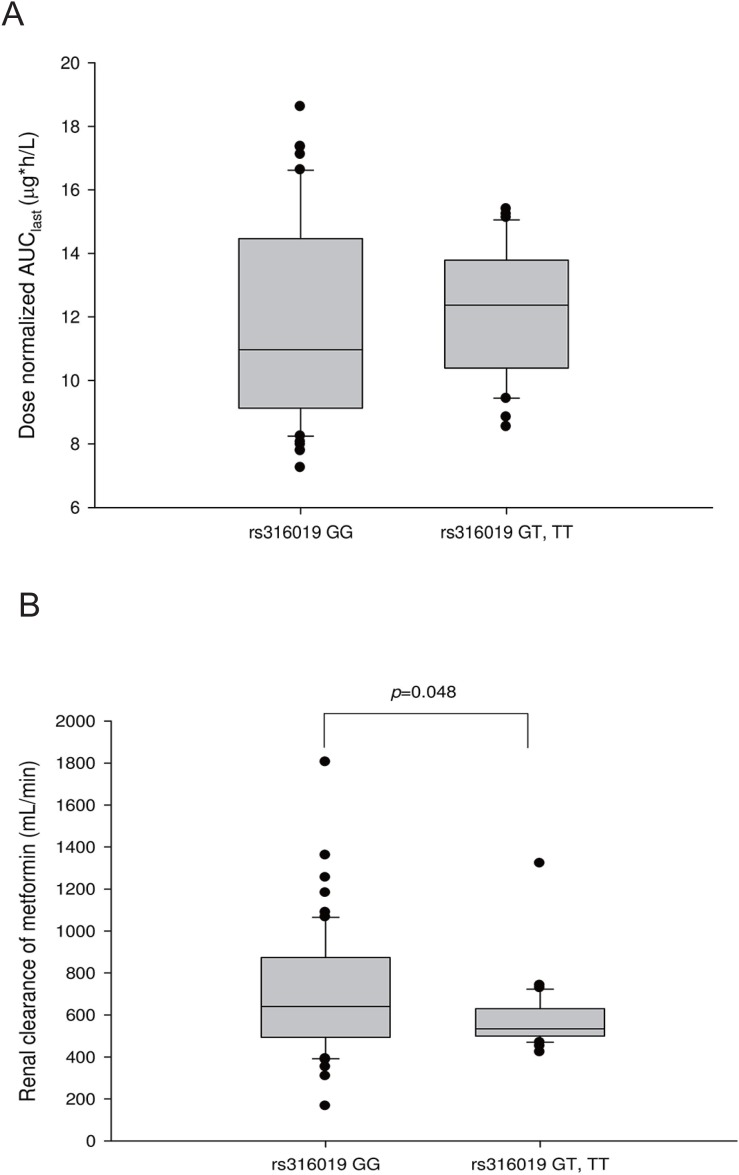
- Metformin, an oral hypoglycemic agent belonging to biguanide class, is widely used to treat type 2 diabetes mellitus, and several drug transporters such as organic cation transporters (OCTs), multidrug and toxin extrusion transporter (MATE), and plasma membrane monoamine transporter (PMAT) are thought to affect its disposition. We evaluated the role of PMAT genetic variations on the pharmacokinetic characteristics of metformin in a Korean population. In this retrospective study, 91 healthy subjects from four different metformin pharmacokinetic studies were analyzed; in each study, the subjects were administered two oral doses of metformin at intervals of 12 hours and dose-normalized pharmacokinetic parameters were compared between the subjects' genotypes. Subjects who had more than one allele of c.883-144A>G single nucleotide polymorphism (SNP) in PMAT gene (rs3889348) showed increased renal clearance of metformin compared to wild-type subjects (814.79 ± 391.73 vs. 619.90 ± 195.43 mL/min, p=0.003), whereas no differences in metformin exposure were observed between the PMAT variant subjects and wild-type subjects. Similarly, subjects with variant rs316019 SNP in OCT2 showed decreased renal clearance of metformin compared to wild-type subjects (586.01 ± 160.54 vs. 699.13 ± 291.40 mL/min, p=0.048). Other SNPs in PMAT and MATE1/2-K genes did not significantly affect metformin pharmacokinetics. In conclusion, the genetic variation of c.883-144A>G SNP in PMAT significantly affects the renal clearance of metformin in healthy Korean male subjects.
MeSH Terms
Figure
Reference
-
1. Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009; 32:193–203. DOI: 10.2337/dc08-9025. PMID: 18945920.2. Gong L, Goswami S, Giacomini KM, Altman RB, Klein TE. Metformin pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2012; 22:820–827. DOI: 10.1097/FPC.0b013e3283559b22. PMID: 22722338.3. Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011; 50:81–98. DOI: 10.2165/11534750-000000000-00000. PMID: 21241070.
Article4. Motohashi H, Sakurai Y, Saito H, Masuda S, Urakami Y, Goto M, et al. Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J Am Soc Nephrol. 2002; 13:866–874. PMID: 11912245.
Article5. Song IS, Shin HJ, Shim EJ, Jung IS, Kim WY, Shon JH, et al. Genetic variants of the organic cation transporter 2 influence the disposition of metformin. Clin Pharmacol Ther. 2008; 84:559–562. DOI: 10.1038/clpt.2008.61. PMID: 18401339.
Article6. Stocker SL, Morrissey KM, Yee SW, Castro RA, Xu L, Dahlin A, et al. The effect of novel promoter variants in MATE1 and MATE2 on the pharmacokinetics and pharmacodynamics of metformin. Clin Pharmacol Ther. 2013; 93:186–194. DOI: 10.1038/clpt.2012.210. PMID: 23267855.
Article7. Wang ZJ, Yin OQ, Tomlinson B, Chow MS. OCT2 polymorphisms and invivo renal functional consequence: studies with metformin and cimetidine. Pharmacogenet Genomics. 2008; 18:637–645. DOI: 10.1097/FPC.0b013e328302cd41. PMID: 18551044.
Article8. Chen Y, Li S, Brown C, Cheatham S, Castro RA, Leabman MK, et al. Effect of genetic variation in the organic cation transporter 2 on the renal elimination of metformin. Pharmacogenet Genomics. 2009; 19:497–504. DOI: 10.1097/FPC.0b013e32832cc7e9. PMID: 19483665.
Article9. Becker ML, Visser LE, van Schaik RH, Hofman A, Uitterlinden AG, Stricker BH. Genetic variation in the multidrug and toxin extrusion 1 transporter protein influences the glucose-lowering effect of metformin in patients with diabetes: a preliminary study. Diabetes. 2009; 58:745–749. DOI: 10.2337/db08-1028. PMID: 19228809.
Article10. Chung JY, Cho SK, Kim TH, Kim KH, Jang GH, Kim CO, et al. Functional characterization of MATE2-K genetic variants and their effects on metformin pharmacokinetics. Pharmacogenet Genomics. 2013; 23:365–373. DOI: 10.1097/FPC.0b013e3283622037. PMID: 23652408.
Article11. Zhou M, Xia L, Wang J. Metformin transport by a newly cloned proton-stimulated organic cation transporter (plasma membrane monoamine transporter) expressed in human intestine. Drug Metab Dispos. 2007; 35:1956–1962. PMID: 17600084.
Article12. Xia L, Engel K, Zhou M, Wang J. Membrane localization and pH-dependent transport of a newly cloned organic cation transporter (PMAT) in kidney cells. Am J Physiol Renal Physiol. 2007; 292:F682–F690. PMID: 17018840.
Article13. Christensen MM, Brasch-Andersen C, Green H, Nielsen F, Damkier P, Beck-Nielsen H, et al. The pharmacogenetics of metformin and its impact on plasma metformin steady-state levels and glycosylated hemoglobin A1c. Pharmacogenet Genomics. 2011; 21:837–850. DOI: 10.1097/FPC.0b013e32834c0010. PMID: 21989078.
Article14. Le Hir H, Nott A, Moore MJ. How introns influence and enhance eukaryotic gene expression. Trends Biochem Sci. 2003; 28:215–220. PMID: 12713906.
Article15. Smith NA, Singh SP, Wang MB, Stoutjesdijk PA, Green AG, Waterhouse PM. Total silencing by intron-spliced hairpin RNAs. Nature. 2000; 407:319–320. PMID: 11014180.
Article16. D'Alessio D. The role of dysregulated glucagon secretion in type 2 diab etes. Diabet Obes Metab. 2011; 13(Suppl 1):S126–S132. DOI: 10.1111/j.1463-1326.2011.01449.x.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pregnane X Receptor agonist Increases the Expression Levels of the Plasma Membrane Monoamine Transporter
- Identification of Novel Genetic Variations in the Proximal Promoter of the Human Transporter, OCT2
- An Association Study between Various Monoamine Transporter Gene Polymorphisms and Treatment Response to Mirtazapine in Major Depression
- Current Understanding in Neurobiology of Depressive Disorders: Imaging Genetic Studies on Serotonin Transporter
- Investigation into the Possible Genetic Role of Serotonin and Dopamine Transporters in Psychological Resilience