Int J Stem Cells.
2018 Jun;11(1):121-130. 10.15283/ijsc18020.
The Role of Microenvironment in Preserving the Potency of Adult Porcine Pulmonary Valve Stem Cells In Vitro
- Affiliations
-
- 1Department of Cardiothoracic Surgery, Division of Pediatric Cardiovascular Surgery, Stanford University, California, USA. fchalajour@gmail.com
- KMID: 2413508
- DOI: http://doi.org/10.15283/ijsc18020
Abstract
- BACKGROUND AND OBJECTIVE
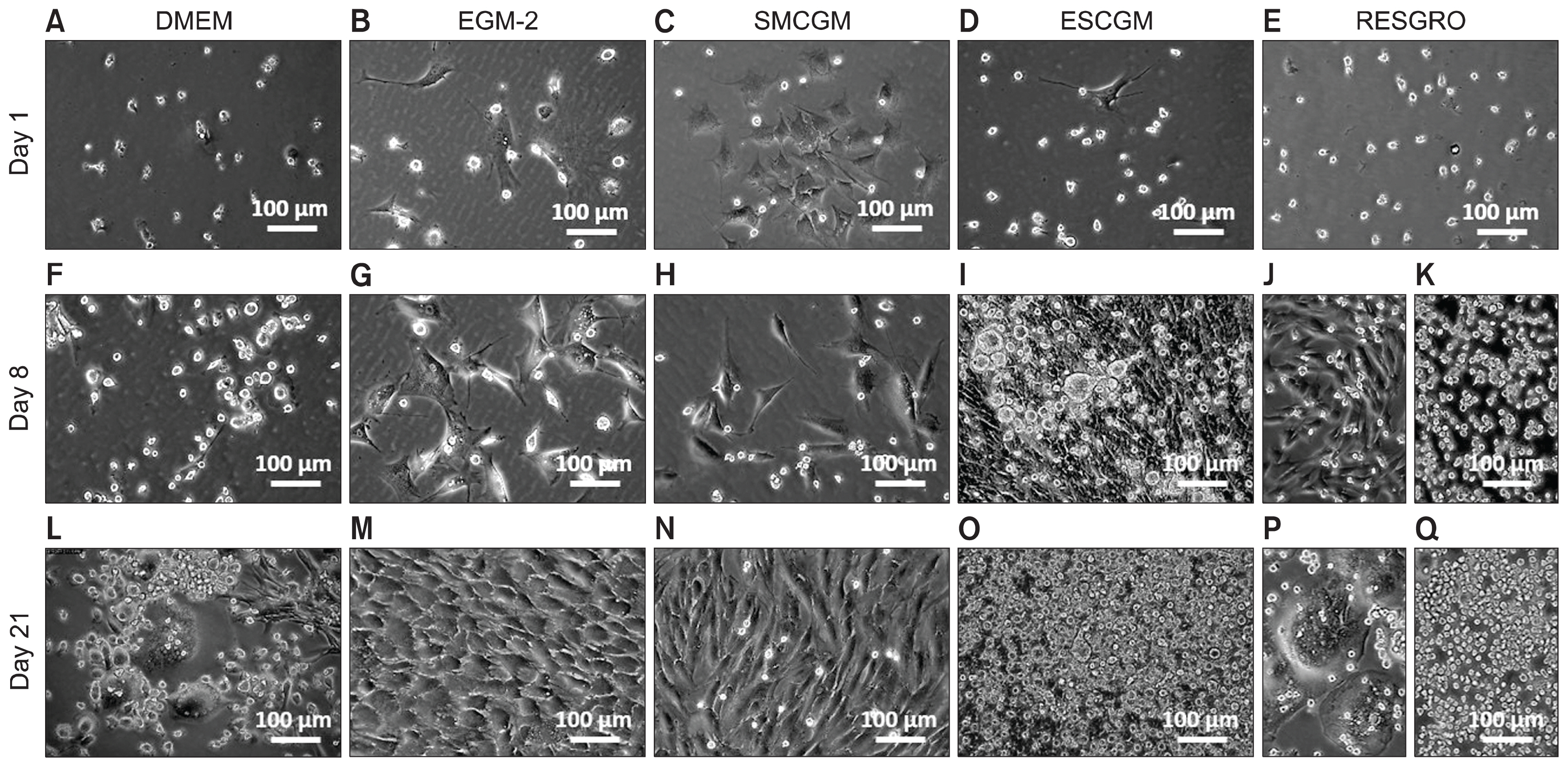
The potency of tissue resident stem cells is regulated primarily by inputs from the local microenvironment. Isolation of stem cells through enzymatic digestion of tissue may affect epigenetic regulation of cell fate and performance. Here we employ a non-enzymatic method to harvest and investigate tissue resident stem cells from the adult porcine pulmonary valve.
METHODS AND RESULTS
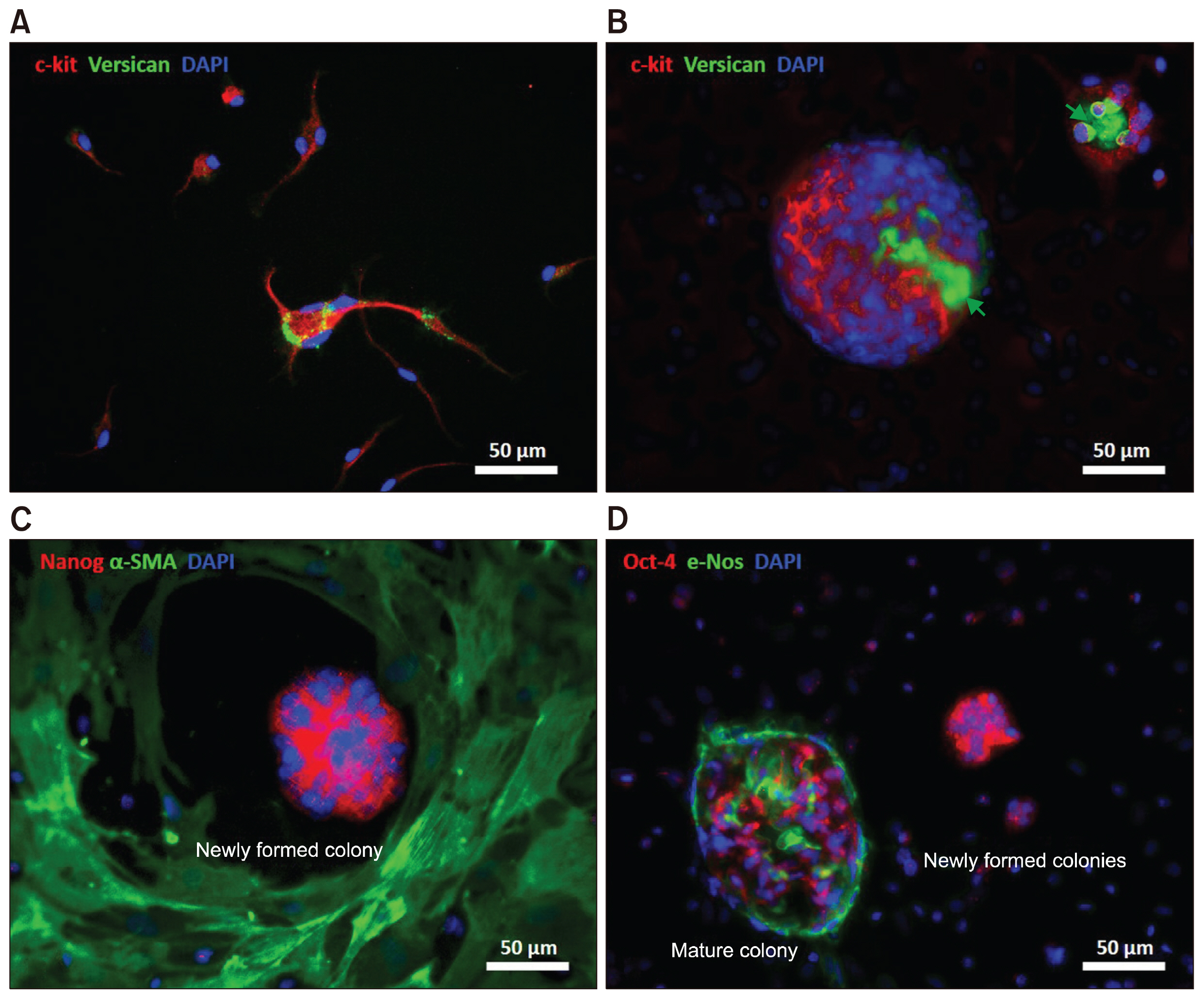
The presence of c-Kit+ stem cells within the valve tissue was confirmed by immunohistochemistry. An in vitro culture of minced valve leaflets was developed under the standard conditions (37°C with 5% CO2). The viability of the cellular outgrowths was evaluated over the subsequent 12 weeks. Under this culture condition, we identified a population of non-adherent c-Kit+ cells and multiple cellular structures mimicking the phenotype of embryonic stem cells at different stages of development. Formation of multinucleated cells through cell fusion provided an active niche area for homing and interaction of the non-adherent c-Kit+ cells. Expression of pluripotency markers Oct-4 and Nanog was detected in the newly formed multinucleated cells but not in mature colonies. Partial cell fusion was shown by fluorescent live-cell tracking, which confirmed intercellular molecular exchange between donor and recipient cells, resulting in altered cytoplasmic protein expression by the recipient cell.
CONCLUSIONS
These results suggest a role for the microenvironment in decrypting the potential of the valve somatic stem cells in vitro. In addition, our data provide evidence for cell fusion, which may play a critical role in reversing somatic cell fate and spontaneous cellular reprogramming.
MeSH Terms
Figure
Reference
-
References
1. Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol. 2008; 9:11–21. DOI: 10.1038/nrm2319.
Article2. Li M, Belmonte JC. Ground rules of the pluripotency gene regulatory network. Nat Rev Genet. 2017; 18:180–191. DOI: 10.1038/nrg.2016.156. PMID: 28045100.
Article3. Avgustinova A, Benitah SA. Epigenetic control of adult stem cell function. Nat Rev Mol Cell Biol. 2016; 17:643–658. DOI: 10.1038/nrm.2016.76. PMID: 27405257.
Article4. Jiang Y, Vaessen B, Lenvik T, Blackstad M, Reyes M, Verfaillie CM. Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp Hematol. 2002; 30:896–904. DOI: 10.1016/S0301-472X(02)00869-X. PMID: 12160841.
Article5. Dubois SG, Floyd EZ, Zvonic S, Kilroy G, Wu X, Carling S, Halvorsen YD, Ravussin E, Gimble JM. Isolation of human adipose-derived stem cells from biopsies and liposuction specimens. Methods Mol Biol. 2008; 449:69–79. PMID: 18370084.
Article6. Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marbán E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007; 115:896–908. DOI: 10.1161/CIRCULATIONAHA.106.655209. PMID: 17283259.
Article7. Mitchell KJ, Pannérec A, Cadot B, Parlakian A, Besson V, Gomes ER, Marazzi G, Sassoon DA. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat Cell Biol. 2010; 12:257–266. PMID: 20118923.
Article8. Lewis FC, Henning BJ, Marazzi G, Sassoon D, Ellison GM, Nadal-Ginard B. Porcine skeletal muscle-derived multipotent PW1pos/Pax7neg interstitial cells: isolation, characterization, and long-term culture. Stem Cells Transl Med. 2014; 3:702–712. DOI: 10.5966/sctm.2013-0174. PMID: 24744394. PMCID: 4039454.
Article9. Toma JG, Akhavan M, Fernandes KJ, Barnabé-Heider F, Sadikot A, Kaplan DR, Miller FD. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001; 3:778–784. DOI: 10.1038/ncb0901-778.
Article10. Paruchuri S, Yang JH, Aikawa E, Melero-Martin JM, Khan ZA, Loukogeorgakis S, Schoen FJ, Bischoff J. Human pulmonary valve progenitor cells exhibit endothelial/mesenchymal plasticity in response to vascular endothelial growth factor-A and transforming growth factor-beta2. Circ Res. 2006; 99:861–869. DOI: 10.1161/01.RES.0000245188.41002.2c. PMID: 16973908. PMCID: 2810464.
Article11. Bischoff J, Aikawa E. Progenitor cells confer plasticity to cardiac valve endothelium. J Cardiovasc Transl Res. 2011; 4:710–719. DOI: 10.1007/s12265-011-9312-0. PMID: 21789724.
Article12. Tauskela JS, Hewitt K, Kang LP, Comas T, Gendron T, Hakim A, Hogan M, Durkin J, Morley P. Evaluation of glutathione-sensitive fluorescent dyes in cortical culture. Glia. 2000; 30:329–341. DOI: 10.1002/(SICI)1098-1136(200006)30:4<329::AID-GLIA20>3.0.CO;2-R. PMID: 10797613.
Article13. Sebastià J, Cristòfol R, Martín M, Rodríguez-Farré E, Sanfeliu C. Evaluation of fluorescent dyes for measuring intracellular glutathione content in primary cultures of human neurons and neuroblastoma SH-SY5Y. Cytometry A. 2003; 51:16–25. DOI: 10.1002/cyto.a.10003.
Article14. Rabkin-Aikawa E, Mayer JE Jr, Schoen FJ. Heart valve regeneration. Adv Biochem Eng Biotechnol. 2005; 94:141–179. PMID: 15915872.
Article15. Liu AC, Joag VR, Gotlieb AI. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol. 2007; 171:1407–1418. DOI: 10.2353/ajpath.2007.070251. PMID: 17823281. PMCID: 2043503.
Article16. Mizuno N, Kosaka M. Novel variants of Oct-3/4 gene expressed in mouse somatic cells. J Biol Chem. 2008; 283:30997–31004. DOI: 10.1074/jbc.M802992200. PMID: 18765667. PMCID: 2662171.
Article17. Lengner CJ, Welstead GG, Jaenisch R. The pluripotency regulator Oct4: a role in somatic stem cells? Cell Cycle. 2008; 7:725–728. DOI: 10.4161/cc.7.6.5573. PMID: 18239456.
Article18. Seymour T, Twigger AJ, Kakulas F. Pluripotency genes and their functions in the normal and aberrant breast and brain. Int J Mol Sci. 2015; 16:27288–27301. DOI: 10.3390/ijms161126024. PMID: 26580604. PMCID: 4661882.
Article19. Selden C, Chalmers SA, Jones C, Standish R, Quaglia A, Rolando N, Burroughs AK, Rolles K, Dhillon A, Hodgson HJ. Epithelial colonies cultured from human explanted liver in subacute hepatic failure exhibit hepatocyte, biliary epithelial, and stem cell phenotypic markers. Stem Cells. 2003; 21:624–631. DOI: 10.1634/stemcells.21-6-624. PMID: 14595121.
Article20. Zeng L, Rahrmann E, Hu Q, Lund T, Sandquist L, Felten M, O’Brien TD, Zhang J, Verfaillie C. Multipotent adult progenitor cells from swine bone marrow. Stem Cells. 2006; 24:2355–2366. DOI: 10.1634/stemcells.2005-0551. PMID: 16931778.
Article21. Gwak JM, Kim M, Kim HJ, Jang MH, Park SY. Expression of embryonal stem cell transcription factors in breast cancer: Oct4 as an indicator for poor clinical outcome and tamoxifen resistance. Oncotarget. 2017; 8:36305–36318. DOI: 10.18632/oncotarget.16750. PMID: 28422735. PMCID: 5482656.
Article22. Villodre ES, Kipper FC, Pereira MB, Lenz G. Roles of OCT4 in tumorigenesis, cancer therapy resistance and prognosis. Cancer Treat Rev. 2016; 51:1–9. DOI: 10.1016/j.ctrv.2016.10.003. PMID: 27788386.
Article23. Müller M, Hermann PC, Liebau S, Weidgang C, Seufferlein T, Kleger A, Perkhofer L. The role of pluripotency factors to drive stemness in gastrointestinal cancer. Stem Cell Res. 2016; 16:349–357. DOI: 10.1016/j.scr.2016.02.005. PMID: 26896855.
Article24. Redshaw Z, Strain AJ. Human haematopoietic stem cells express Oct4 pseudogenes and lack the ability to initiate Oct4 promoter-driven gene expression. J Negat Results Biomed. 2010; 9:2. DOI: 10.1186/1477-5751-9-2. PMID: 20356403. PMCID: 2853495.
Article25. Liedtke S, Enczmann J, Waclawczyk S, Wernet P, Kögler G. Oct4 and its pseudogenes confuse stem cell research. Cell Stem Cell. 2007; 1:364–366. DOI: 10.1016/j.stem.2007.09.003.
Article26. Lengner CJ, Camargo FD, Hochedlinger K, Welstead GG, Zaidi S, Gokhale S, Scholer HR, Tomilin A, Jaenisch R. Oct4 expression is not required for mouse somatic stem cell self-renewal. Cell Stem Cell. 2007; 1:403–415. DOI: 10.1016/j.stem.2007.07.020. PMID: 18159219. PMCID: 2151746.
Article27. Dyce PW, Wen L, Li J. In vitro germline potential of stem cells derived from fetal porcine skin. Nat Cell Biol. 2006; 8:384–390. DOI: 10.1038/ncb1388. PMID: 16565707.
Article28. Ma Z, Hu Y, Jiang G, Hou J, Liu R, Lu Y, Liu C. Spontaneous generation of germline characteristics in mouse fibrosarcoma cells. Sci Rep. 2012; 2:743. DOI: 10.1038/srep00743. PMID: 23077727. PMCID: 3473365.
Article29. Dyce PW, Shen W, Huynh E, Shao H, Villagómez DA, Kidder GM, King WA, Li J. Analysis of oocyte-like cells differentiated from porcine fetal skin-derived stem cells. Stem Cells Dev. 2011; 20:809–819. DOI: 10.1089/scd.2010.0395.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Glutathione Dynamics in the Tumor Microenvironment: A Potential Target of Cancer Stem Cells and T Cells
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Bioreactor Conditioning of Valve Scaffolds Seeded Internally with Adult Stem Cells
- Endogenous Stem Cells in the Ear
- Measurement of Porcine Aortic and Pulmonary Valve Geometry and Design for Implantable Tissue Valve