Blood Res.
2017 Jun;52(2):100-105. 10.5045/br.2017.52.2.100.
Intrachromosomal amplification of chromosome 21 in Korean pediatric patients with B-cell precursor acute lymphoblastic leukemia in a single institution
- Affiliations
-
- 1Department of Laboratory Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. sunnyhk@skku.edu
- 2Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2413326
- DOI: http://doi.org/10.5045/br.2017.52.2.100
Abstract
- BACKGROUND
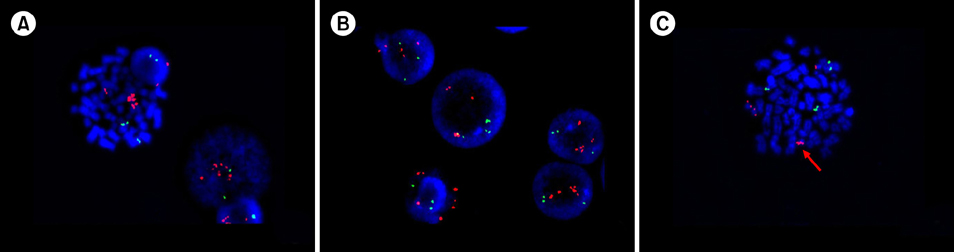
Intrachromosomal amplification of chromosome 21 (iAMP21), defined as the presence of three or more RUNX1 signals on one marker chromosome, is a distinct cytogenetic subgroup of childhood B-cell precursor acute lymphoblastic leukemia (BCP-ALL) that is known to have a poor prognosis when treated with standard therapy. The aim of this study was to evaluate the clinical characteristics of Korean children with iAMP21.
METHODS
The cytogenetic data from BCP-ALL children were reviewed. The ETV6/RUNX1 ES Dual Color Probe was used for fluorescence in situ hybridization (FISH).
RESULTS
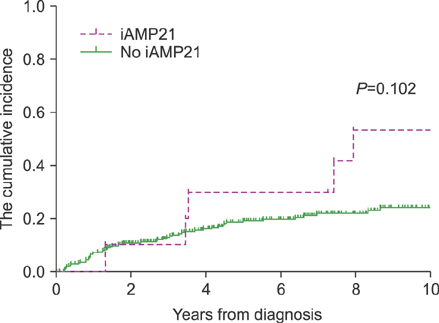
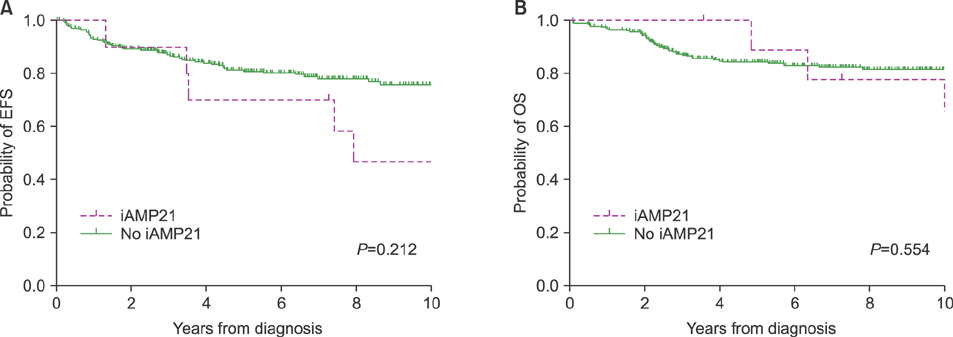
In total, 295 children were included. Of these, 10 patients (3.4%) had iAMP21. The median age of iAMP21 patients was 9 years, and the median value of white blood cell count was 5.09×10â¹/L. Slow early treatment response was observed more in iAMP21 patients. Patients with iAMP21 had a higher incidence of relapse and worse survival rates. In patients with iAMP21, the estimated 10-year cumulative incidence of relapse was 53.3%. The estimated 10-year event-free survival and overall survival rate were 46.7% and 64.8%, respectively. Most cases of leukemic relapse developed in the late period (median, 43 mo). In multivariate analysis, high risk group was the only factor that had a significant impact on death.
CONCLUSION
The existence of iAMP21 was related to delayed treatment response and was likely to affect increased relapse and death in the late period. Further studies are needed to reveal its effect on BCP-ALL treatment outcomes and its role as an independent prognostic factor.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Optimal therapy for adolescents and young adults with acute lymphoblastic leukemia-current perspectives
Jae Wook Lee
Blood Res. 2020;55(Supplement):S27-S31. doi: 10.5045/br.2020.S005.Optimal therapy for adolescents and young adults with acute lymphoblastic leukemia-current perspectives
Jae Wook Lee
Blood Res. 2020;55(S1):S27-S31. doi: 10.5045/br.2020.S005.
Reference
-
1. Harewood L, Robinson H, Harris R, et al. Amplification of AML1 on a duplicated chromosome 21 in acute lymphoblastic leukemia: a study of 20 cases. Leukemia. 2003; 17:547–553.
Article2. Soulier J, Trakhtenbrot L, Najfeld V, et al. Amplification of band q22 of chromosome 21, including AML1, in older children with acute lymphoblastic leukemia: an emerging molecular cytogenetic subgroup. Leukemia. 2003; 17:1679–1682.
Article3. Harrison CJ. Blood Spotlight on iAMP21 acute lymphoblastic leukemia (ALL), a high-risk pediatric disease. Blood. 2015; 125:1383–1386.
Article4. Harrison CJ, Haas O, Harbott J, et al. Detection of prognostically relevant genetic abnormalities in childhood B-cell precursor acute lymphoblastic leukaemia: recommendations from the Biology and Diagnosis Committee of the International Berlin-Frankfürt-Münster study group. Br J Haematol. 2010; 151:132–142.
Article5. Moorman AV, Robinson H, Schwab C, et al. Risk-directed treatment intensification significantly reduces the risk of relapse among children and adolescents with acute lymphoblastic leukemia and intrachromosomal amplification of chromosome 21: a comparison of the MRC ALL97/99 and UKALL2003 trials. J Clin Oncol. 2013; 31:3389–3396.
Article6. Heerema NA, Carroll AJ, Devidas M, et al. Intrachromosomal amplification of chromosome 21 is associated with inferior outcomes in children with acute lymphoblastic leukemia treated in contemporary standard-risk children's oncology group studies: a report from the children's oncology group. J Clin Oncol. 2013; 31:3397–3402.
Article7. Moorman AV, Richards SM, Robinson HM, et al. Prognosis of children with acute lymphoblastic leukemia (ALL) and intrachromosomal amplification of chromosome 21 (iAMP21). Blood. 2007; 109:2327–2330.
Article8. Robinson HM, Harrison CJ, Moorman AV, Chudoba I, Strefford JC. Intrachromosomal amplification of chromosome 21 (iAMP21) may arise from a breakage-fusion-bridge cycle. Genes Chromosomes Cancer. 2007; 46:318–326.
Article9. Harrison CJ, Moorman AV, Schwab C, et al. An international study of intrachromosomal amplification of chromosome 21 (iAMP21): cytogenetic characterization and outcome. Leukemia. 2014; 28:1015–1021.
Article10. Johnson RC, Weinberg OK, Cascio MJ, et al. Cytogenetic variation of B-lymphoblastic leukemia with Intrachromosomal Amplification of Chromosome 21 (iAMP21): A multi-institutional series review. Am J Clin Pathol. 2015; 144:103–112.
Article11. Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children's oncology group. J Clin Oncol. 2012; 30:1663–1669.
Article12. Gaynon PS, Angiolillo AL, Carroll WL, et al. Long-term results of the children's cancer group studies for childhood acute lymphoblastic leukemia 1983-2002: a Children's Oncology Group Report. Leukemia. 2010; 24:285–297.
Article13. Attarbaschi A, Mann G, Panzer-Grümayer R, et al. Minimal residual disease values discriminate between low and high relapse risk in children with B-cell precursor acute lymphoblastic leukemia and an intrachromosomal amplification of chromosome 21: the Austrian and German acute lymphoblastic leukemia Berlin-Frankfurt-Munster (ALL-BFM) trials. J Clin Oncol. 2008; 26:3046–3050.
Article14. Attarbaschi A, Panzer-Grümayer R, Mann G, et al. Minimal residual disease-based treatment is adequate for relapse-prone childhood acute lymphoblastic leukemia with an intrachromosomal amplification of chromosome 21: the experience of the ALL-BFM 2000 trial. Klin Padiatr. 2014; 226:338–343.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Precursor B-Cell Acute Lymphoblastic Leukemia in Two Patients with a History of Cytotoxic Therapy
- Mixed-phenotypic acute leukemia: cytochemically myeloid and phenotypically early T-cell precursor acute lymphoblastic leukemia
- A Case of Acute Lymphoblastic Leukemia with ider(9)(q10)t(9;22)(q34;q11.2)
- A Case of Pediatric Acute Lymphoblastic Leukemia with Trisomy 5 as a Sole Chromosomal Anomaly: A Prognostic Significance
- The e1a3 BCR-ABL1 Fusion Transcript in Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia