J Vet Sci.
2016 Jun;17(2):145-152. 10.4142/jvs.2016.17.2.145.
Improved development of somatic cell cloned bovine embryos by a mammary gland epithelia cells in vitro model
- Affiliations
-
- 1School of Life Science and Technology, Inner Mongolia University of Science & Technology, Baotou 014010, China. hxy1124@163.com
- 2Research and Development Center for Tissue Engineering, Xi'an 710048, China.
- 3College of Veterinary Medicine, Northwest A&F University, Shenyang 712100, China.
- 4Key Laboratory of Animal Reproductive Endocrinology & Embryo Engineering, Ministry of Agriculture, Shenyang 712100, China.
- KMID: 2413163
- DOI: http://doi.org/10.4142/jvs.2016.17.2.145
Abstract
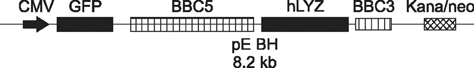
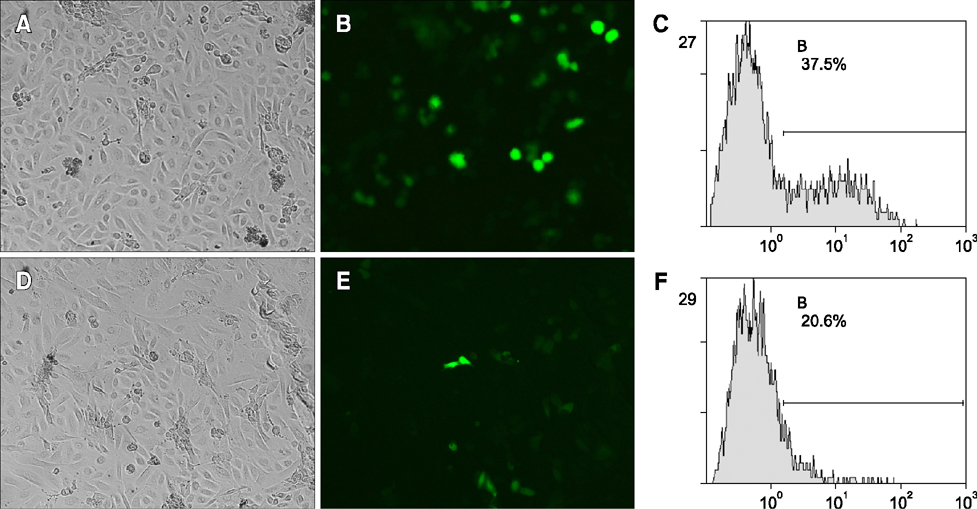
- Previous studies have established a bovine mammary gland epithelia cells in vitro model by the adenovirus-mediated telomerase (hTERT-bMGEs). The present study was conducted to confirm whether hTERT-bMGEs were effective target cells to improve the efficiency of transgenic expression and somatic cell nuclear transfer (SCNT). To accomplish this, a mammary-specific vector encoding human lysozyme and green fluorescent protein was used to verify the transgenic efficiency of hTERT-bMGEs, and untreated bovine mammary gland epithelial cells (bMGEs) were used as a control group. The results showed that the hTERT-bMGEs group had much higher transgenic efficiency and protein expression than the bMGEs group. Furthermore, the nontransgenic and transgenic hTERT-bMGEs were used as donor cells to evaluate the efficiency of SCNT. There were no significant differences in rates of cleavage or blastocysts or hatched blastocysts of cloned embryos from nontransgenic hTERT-bMGEs at passage 18 and 28 groups (82.8% vs. 81.9%, 28.6% vs. 24.8%, 58.6% vs. 55.3%, respectively) and the transgenic group (80.8%, 26.5% and 53.4%); however, they were significantly higher than the bMGEs group (71.2%, 12.8% and 14.8%), (p < 0.05). We confirmed that hTERT-bMGEs could serve as effective target cells for improving development of somatic cell cloned cattle embryos.
Keyword
MeSH Terms
-
Adenoviridae/genetics
Animals
Animals, Genetically Modified/genetics/metabolism
Cattle
Cloning, Organism/veterinary
Embryonic Development
Female
*Gene Expression
Green Fluorescent Proteins/genetics/metabolism
Mammary Glands, Animal/cytology/*metabolism
Muramidase/*genetics/metabolism
Nuclear Transfer Techniques/*veterinary
Recombinant Proteins/genetics/metabolism
Telomerase/*biosynthesis/genetics
Transfection/veterinary
Recombinant Proteins
Green Fluorescent Proteins
Telomerase
Muramidase
Figure
Reference
-
1. Arat S, Gibbons J, Rzucidlo SJ, Respess DS, Tumlin M, Stice SL. In vitro development of bovine nuclear transfer embryos from transgenic clonal lines of adult and fetal fibroblast cells of the same genotype. Biol Reprod. 2002; 66:1768–1774.
Article2. Betthauser J, Forsberg E, Augenstein M, Childs L, Eilertsen K, Enos J, Forsythe T, Golueke P, Jurgella G, Koppang R, Lesmeister T, Mallon K, Mell G, Misica P, Pace M, Pfister-Genskow M, Strelchenko N, Voelker G, Watt S, Thompson S, Bishop M. Production of cloned pigs from in vitro systems. Nat Biotechnol. 2000; 18:1055–1059.
Article3. Bonnet-Garnier A, Veillard AC, Bed'Hom B, Hayes H, Britton-Davidian J. Assessing the quality of donor cells: karyotyping methods. Methods Mol Biol. 2015; 1222:83–99.
Article4. Bressan FF, Sangalli JR, Pessôa LVF, Pires PRL, Meirelles FV. Insights on bovine genetic engineering and cloning. Pesqui Vet Bras. 2013; 33:Suppl. 113–118.
Article5. Brophy B, Smolenski G, Wheeler T, Wells D, L'Huillier P, Laible G. Cloned transgenic cattle produce milk with higher levels of β-casein and κ-casein. Nat Biotechnol. 2003; 21:157–162.
Article6. Campbell KHS, Fisher P, Chen WC, Choi I, Kelly RDW, Lee JH, Xhu J. Somatic cell nuclear transfer: past, present and future perspectives. Theriogenology. 2007; 68:Suppl 1. S214–S231.
Article7. Carvalho AV, Reinaud P, Forde N, Healey GD, Eozenou C, Giraud-Delville C, Mansouri-Attia N, Gall L, Richard C, Lonergan P, Sheldon IM, Lea RG, Sandra O. SOCS genes expression during physiological and perturbed implantation in bovine endometrium. Reproduction. 2014; 148:545–557.8. Firas J, Liu XD, Polo JM. Epigenetic memory in somatic cell nuclear transfer and induced pluripotency: evidence and implications. Differentiation. 2014; 88:29–32.
Article9. Gibson CA, Vega JR, Baumrucker CR, Oakley CS, Welsch CW. Establishment and characterization of bovine mammary epithelial cell lines. In Vitro Cell Dev Biol. 1991; 27:585–594.
Article10. He XY, Zheng YM, Lan J, Wu YH, Yan J, He XN, Zhang T, He YL, Zheng YL, Zhang Y. Recombinant adenovirus-mediated human telomerase reverse transcriptase gene can stimulate cell proliferation and maintain primitive characteristics in bovine mammary gland epithelial cells. Dev Growth Differ. 2011; 53:312–322.
Article11. Heyman Y, Chavatte-Palmer P, LeBourhis D, Camous S, Vignon X, Renard JP. Frequency and occurrence of late-gestation losses from cattle cloned embryos. Biol Reprod. 2002; 66:6–13.
Article12. Hodges CA, Stice SL. Generation of bovine transgenics using somatic cell nuclear transfer. Reprod Biol Endocrinol. 2003; 1:81.13. Kang YK, Koo DB, Park JS, Choi YH, Chung AS, Lee KK, Han YK. Aberrant methylation of donor genome in cloned bovine embryos. Nat Genet. 2001; 28:173–177.
Article14. Kang YK, Yeo S, Kim SH, Koo DB, Park JS, Wee G, Han JS, Oh KB, Lee KK, Han YM. Precise recapitulation of methylation change in early cloned embryos. Mol Reprod Dev. 2003; 66:32–37.
Article15. Kato Y, Tani T, Tsunoda Y. Cloning of calves from various somatic cell types of male and female adult, newborn and fetal cows. J Reprod Fertil. 2000; 120:231–237.
Article16. Kung MH, Lee YJ, Hsu JT, Huang MC, Ju YT. A functional study of proximal goat β-casein promoter and intron 1 in immortalized goat mammary epithelial cells. J Dairy Sci. 2015; 98:3859–3875.
Article17. Kurome M, Kessler B, Wuensch A, Nagashima H, Wolf E. Nuclear transfer and transgenesis in the pig. Methods Mol Biol. 2015; 1222:37–59.
Article18. Liu J, Luo Y, Liu Q, Zheng L, Yang Z, Wang Y, Su J, Quan F, Zhang Y. Production of cloned embryos from caprine mammary epithelial cells expressing recombinant human β-defensin-3. Theriogenology. 2013; 79:660–666.
Article19. Luchsinger C, Arias ME, Vargas T, Paredes M, Sánchez R, Felmer R. Stability of reference genes for normalization of reverse transcription quantitative real-time PCR (RT-qPCR) data in bovine blastocysts produced by IVF, ICSI and SCNT. Zygote. 2014; 22:505–512.
Article20. Ma LB, Cai L, Li JJ, Chen XL, Ji FY. Two-staged nuclear transfer can enhance the developmental ability of goat-sheep interspecies nuclear transfer embryos in vitro. In Vitro Cell Dev Biol Anim. 2011; 47:95–103.
Article21. Martins LT, Gaudencio Neto S, Aguiar LH, Calderón CEM, Tavares KCS, Carneiro IS, Morais AS, Girao Neto FXA, Pinho RM, Almeida AP, Lazzarotto CR, Chies JM, Bertolini LR, Forell F, Bertolini M. 37 Effect of cell manipulation for production of transgenic cell lines on goat cloning efficiency. Reprod Fertil Dev. 2014; 27:111.
Article22. Matoba S, Liu Y, Lu F, Iwabuchi KA, Shen L, Inoue A, Zhang Y. Embryonic development following somatic cell nuclear transfer impeded by persisting histone methylation. Cell. 2014; 159:884–895.
Article23. Mizutani E, Wakayama S, Wakayama T. Treatment of donor cell/embryo with different approaches to improve development after nuclear transfer. Methods Mol Biol. 2015; 1222:101–111.
Article24. Niemann H, Tian XC, King WA, Lee RS. Epigenetic reprogramming in embryonic and foetal development upon somatic cell nuclear transfer cloning. Reproduction. 2008; 135:151–163.
Article25. Onishi A, Iwamoto M, Akita T, Mikawa S, Takeda K, Awata T, Hanada H, Perry AC. Pig cloning by microinjection of fetal fibroblast nuclei. Science. 2000; 289:1188–1190.
Article26. Park HJ, Koo OJ, Kwon DK, Kang JT, Jang G, Lee BC. Effect of roscovitine-treated donor cells on development of porcine cloned embryos. Reprod Domest Anim. 2010; 45:1082–1088.
Article27. Polejaeva IA, Chen SH, Vaught TD, Page RL, Mullins J, Ball S, Dai Y, Boone J, Walker S, Ayares DL, Colman A, Campbell KHS. Cloned pigs produced by nuclear transfer from adult somatic cells. Nature. 2000; 407:86–90.
Article28. Santos F, Zakhartchenko V, Stojkovic M, Peters A, Jenuwein T, Wolf E, Reik W, Dean W. Epigenetic marking correlates with developmental potential in cloned bovine preimplantation embryos. Curr Biol. 2003; 13:1116–1121.
Article29. Shi W, Hoeflich A, Flaswinkel H, Stojkovic M, Wolf E, Zakhartchenko V. Induction of a senescent-like phenotype does not confer the ability of bovine immortal cells to support the development of nuclear transfer embryos. Biol Reprod. 2003; 69:301–309.
Article30. Su J, Hu G, Wang Y, Liang D, Gao M, Sun H, Zhang Y. Recombinant human growth differentiation factor-9 improves oocyte reprogramming competence and subsequent development of bovine cloned embryos. Cell Reprogram. 2014; 16:281–289.
Article31. Wang Y, Zhao S, Bai L, Fan J, Liu E. Expression systems and species used for transgenic animal bioreactors. Biomed Res Int. 2013; 2013:580463.
Article32. Yang R, Liu J, An Z, Li Z, Zhang Y. Cloning of bovine beta-casein gene and construction of mammary gland-specific expression vector. Zhongguo Sheng Wu Gong Cheng Za Zhi. 2005; 1:260–263.33. Zakhartchenko V, Alberio R, Stojkovic M, Prelle K, Schernthaner W, Stojkovic P, Wenigerkind H, Wanke R, Düchler M, Steinborn R, Mueller M, Brem G, Wolf E. Adult cloning in cattle: potential of nuclei from a permanent cell line and from primary cultures. Mol Reprod Dev. 1999; 54:264–272.
Article34. Zakhartchenko V, Mueller S, Alberio R, Schernthaner W, Stojkovic M, Wenigerkind H, Wanke R, Lassnig C, Mueller M, Wolf E, Brem G. Nuclear transfer in cattle with non-transfected and transfected fetal or cloned transgenic fetal and postnatal fibroblasts. Mol Reprod Dev. 2001; 60:362–369.
Article35. Zheng YM, An ZX, Peng XR, Shi YQ, Zhang Y. EGFP expression in goat mammary epithelial cells. Chin J Agric Biotechnol. 2006; 3:71–74.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Bovine Serum Albumin on in Vitro Growth and Development of Mouse Two-Cell Stage Embryos
- Evaluation of porcine urine-derived cells as nuclei donor for somatic cell nuclear transfer
- Cloned calves derived from somatic cell nuclear transfer embryos cultured in chemically defined medium or modified synthetic oviduct fluid
- The Effect of Bovine Serum Albumin on in-Vitro Growth and Development of Mouse Two-Cell Stage Embryos Fertilized in-Vivo
- Production of cloned sei whale (Balaenoptera borealis) embryos by interspecies somatic cell nuclear transfer using enucleated pig oocytes