Ann Surg Treat Res.
2018 Jun;94(6):306-311. 10.4174/astr.2018.94.6.306.
Additional 4-week capecitabine during the resting periods after 6-week neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer: long-term oncologic outcomes
- Affiliations
-
- 1Department of Surgery, Chungnam National University Hospital, Daejeon, Korea. lllllkh@cnuh.co.kr
- KMID: 2412400
- DOI: http://doi.org/10.4174/astr.2018.94.6.306
Abstract
- PURPOSE
The aim of this study was to evaluate the long-term outcome of additional 4-week chemotherapy with capecitabine during the resting periods following a 6-week neoadjuvant chemoradiotherapy (NCRT) regimen, in patients with locally advanced rectal cancer.
METHODS
Radiotherapy was delivered to the whole pelvis at a total dose of 50.4 Gy for 6 weeks. Oral capecitabine was administered at a dose of 825 mg/m2 twice daily for 10 weeks. Surgery was performed 2-4 weeks following the completion of chemotherapy.
RESULTS
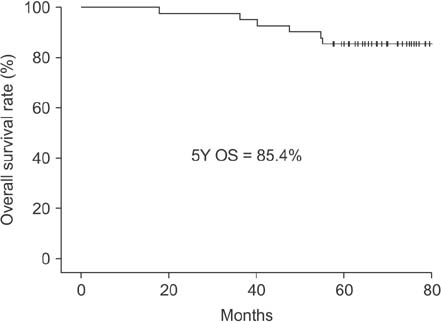
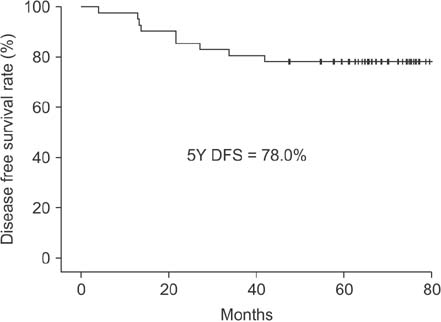
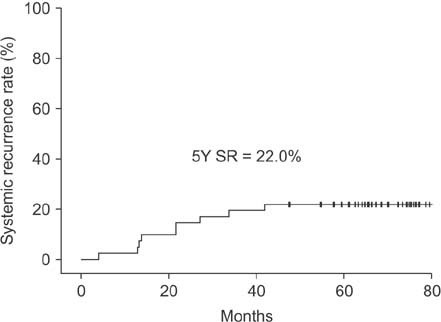
Between January 2010 and September 2011, 41 patients completed the scheduled neoadjuvant therapy and surgery. The pathologic complete response rate, 5-year overall survival, and 5-year disease-free survival rates were 22%, 85.4%, and 78.0%, respectively. The 5-year systemic recurrence and 5-year local recurrence rates were 22% and 0%, respectively.
CONCLUSION
Additional 4-week chemotherapy with capecitabine, during the resting periods following a 6-week NCRT regimen, has favorable long-term oncologic outcomes. Further randomized controlled trials are however necessary to evaluate if substantial improvement in local control is achieved with this additional chemotherapy modality for locally advanced rectal cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006; 355:1114–1123.
Article2. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines): Colon/rectal cancer. ver. 1. 2010 [Internet]. Fort Wathington (PA): National Comprehensive Cancer Network;c2017. cited 2017 Jul 19. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.3. Rodel C, Graeven U, Fietkau R, Hohenberger W, Hothorn T, Arnold D, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015; 16:979–989.4. Gruenberger B, Tamandl D, Schueller J, Scheithauer W, Zielinski C, Herbst F, et al. Bevacizumab, capecitabine, and oxaliplatin as neoadjuvant therapy for patients with potentially curable metastatic colorectal cancer. J Clin Oncol. 2008; 26:1830–1835.
Article5. Eisterer W, Piringer G, DE Vries A, Ofner D, Greil R, Tschmelitsch J, et al. Neoadjuvant chemotherapy with capecitabine, oxaliplatin and bevacizumab followed by concomitant chemoradiation and surgical resection in locally advanced rectal cancer with high risk of recurrence - A Phase II Study. Anticancer Res. 2017; 37:2683–2691.6. Habr-Gama A, Perez RO, Sao Juliao GP, Proscurshim I, Fernandez LM, Figueiredo MN, et al. Consolidation chemotherapy during neoadjuvant chemoradiation (CRT) for distal rectal cancer leads to sustained decrease in tumor metabolism when compared to standard CRT regimen. Radiat Oncol. 2016; 11:24.
Article7. Maas M, Nelemans PJ, Valentini V, Das P, Rodel C, Kuo LJ, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010; 11:835–844.
Article8. Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003; 300:1155–1159.
Article9. Mueller AK, Lindner K, Hummel R, Haier J, Watson DI, Hussey DJ. MicroRNAs and their impact on radiotherapy for cancer. Radiat Res. 2016; 185:668–677.
Article10. Li L, Story M, Legerski RJ. Cellular responses to ionizing radiation damage. Int J Radiat Oncol Biol Phys. 2001; 49:1157–1162.
Article11. Pawlik TM, Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys. 2004; 59:928–942.
Article12. Lawrence TS, Blackstock AW, McGinn C. The mechanism of action of radiosensitization of conventional chemotherapeutic agents. Semin Radiat Oncol. 2003; 13:13–21.
Article13. Hofheinz RD, Wenz F, Post S, Matzdorff A, Laechelt S, Hartmann JT, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol. 2012; 13:579–588.
Article14. Byfield JE. Useful interactions between 5-fluorouracil and radiation in man: 5-fluorouracil as a radiosensitizer. In : Hill BT, Bellamy AS, editors. Antitumor drugradiation interactions. Stuttgart (NY): CRC Press, Inc.;1990. p. 87–105.15. Kim JS, Kim JS, Cho MJ, Song KS, Yoon WH. Preoperative chemoradiation using oral capecitabine in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2002; 54:403–408.
Article16. Gerard JP, Azria D, Gourgou-Bourgade S, Martel-Laffay I, Hennequin C, Etienne PL, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010; 28:1638–1644.17. Aschele C, Cionini L, Lonardi S, Pinto C, Cordio S, Rosati G, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011; 29:2773–2780.18. O'Connell MJ, Colangelo LH, Beart RW, Petrelli NJ, Allegra CJ, Sharif S, et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R-04. J Clin Oncol. 2014; 32:1927–1934.19. Schmoll HJ, Haustermans K, Price TJ, Nordlinger B, Hofheinz R, Daisne JF, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with capecitabine and oxaliplatin versus capecitabine alone in locally advanced rectal cancer: disease-free survival results at interim analysis. J Clin Oncol. 2014; 32:15 Suppl. 3501.20. Rodel C, Liersch T, Becker H, Fietkau R, Hohenberger W, Hothorn T, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012; 13:679–687.21. De Felice F, Benevento I, Magnante AL, Musio D, Bulzonetti N, Caiazzo R, et al. Clinical benefit of adding oxaliplatin to standard neoadjuvant chemoradiotherapy in locally advanced rectal cancer: a meta-analysis : oxaliplatin in neoadjuvant treatment for rectal cancer. BMC Cancer. 2017; 17:325.
Article22. Fu XL, Fang Z, Shu LH, Tao GQ, Wang JQ, Rui ZL, et al. Meta-analysis of oxaliplatin-based versus fluorouracil-based neoadjuvant chemoradiotherapy and adjuvant chemotherapy for locally advanced rectal cancer. Oncotarget. 2017; 8:34340–34351.
Article23. Fornaro L, Caparello C, Vivaldi C, Rotella V, Musettini G, Falcone A, et al. Bevacizumab in the pre-operative treatment of locally advanced rectal cancer: a systematic review. World J Gastroenterol. 2014; 20:6081–6091.
Article24. Weiss C, Arnold D, Dellas K, Liersch T, Hipp M, Fietkau R, et al. Preoperative radiotherapy of advanced rectal cancer with capecitabine and oxaliplatin with or without cetuximab: A pooled analysis of three prospective phase I-II trials. Int J Radiat Oncol Biol Phys. 2010; 78:472–478.
Article25. Francois Y, Nemoz CJ, Baulieux J, Vignal J, Grandjean JP, Partensky C, et al. Influence of the interval between preoperative radiation therapy and surgery on down-staging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. 1999; 17:2396.
Article26. Sloothaak DA, Geijsen DE, van Leersum NJ, Punt CJ, Buskens CJ, Bemelman WA, et al. Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg. 2013; 100:933–939.
Article27. Macchia G, Gambacorta MA, Masciocchi C, Chiloiro G, Mantello G, di Benedetto M, et al. Time to surgery and pathologic complete response after neoadjuvant chemoradiation in rectal cancer: a population study on 2094 patients. Clin Transl Radiat Oncol. 2017; 4:8–14.
Article28. Smith FM, Ahad A, Perez RO, Marks J, Bujko K, Heald RJ. Local excision techniques for rectal cancer after neoadjuvant chemoradiotherapy: what are we doing? Dis Colon Rectum. 2017; 60:228–239.
Article29. Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015; 16:957–966.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Phase II Study of Additional Four-Week Chemotherapy With Capecitabine During the Resting Periods After Six-Week Neoadjuvant Chemoradiotherapy in Patients With Locally Advanced Rectal Cancer
- Long-term oncologic outcomes of neoadjuvant concurrent chemoradiotherapy with capecitabine and radical surgery in locally advanced rectal cancer: 10-year experiences at a single institution
- Long-term oncologic outcomes in pathologic tumor response after neoadjuvant chemoradiation for locally advanced rectal cancer
- Early Systemic Failure After Preoperative Chemoradiotherapy for the Treatment of Patients With Rectal Cancer
- Organ Preservation Strategies After Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer