Ann Surg Treat Res.
2016 Oct;91(4):178-186. 10.4174/astr.2016.91.4.178.
Long-term oncologic outcomes of neoadjuvant concurrent chemoradiotherapy with capecitabine and radical surgery in locally advanced rectal cancer: 10-year experiences at a single institution
- Affiliations
-
- 1Department of Surgery, Chungnam National University Hospital, Daejeon, Korea. jkim@cnu.ac.kr
- KMID: 2354325
- DOI: http://doi.org/10.4174/astr.2016.91.4.178
Abstract
- PURPOSE
Oral capecitabine has demonstrated to be safe and efficient as neoadjuvant concurrent chemoradiotherapy (NCRT) for locally advanced rectal cancers. The aim of this study was to evaluate the long-term oncologic outcomes of NCRT with capecitabine and radical surgery.
METHODS
From January 2000 to June 2010, 238 patients were treated at our center for locally advanced rectal cancers using conventional NCRT with capecitabine and radical surgery. Univariate and multivariate analyses were used to evaluate the factors associated with oncologic outcomes with log rank and Cox regression tests.
RESULTS
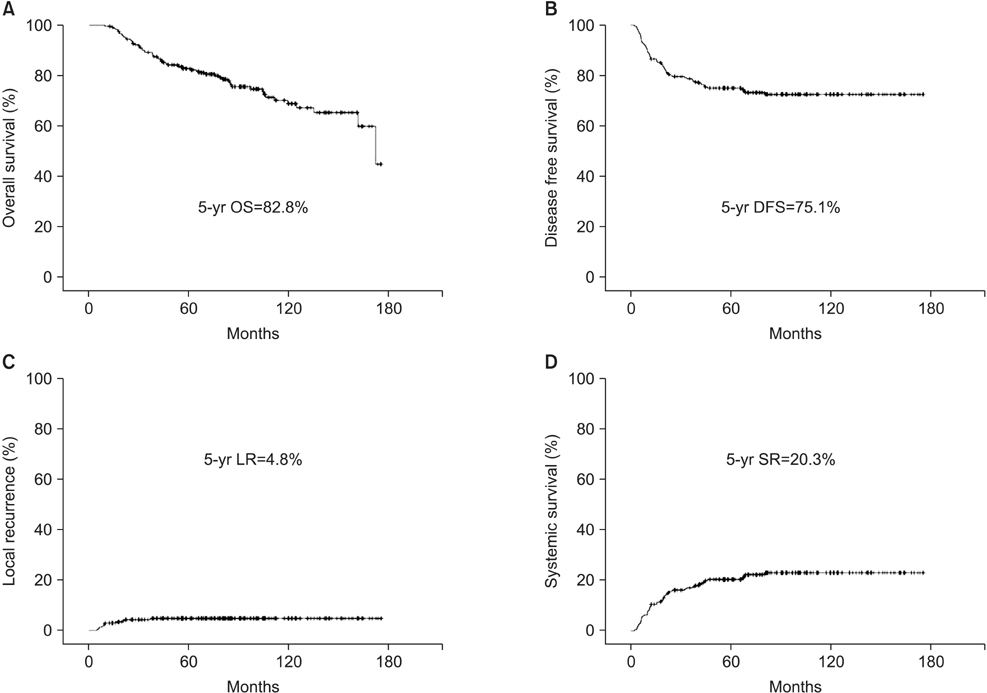
The incidence of grade >3 capecitabine-related toxicity was found to be 4.6%. A pathologic complete response was observed in 14.7% of patients. The 5-year overall and 5-year disease-free survival rate, local and systemic recurrence rate were 82.8%, 75.1%, 4.8%, and 20.3%. Abdominoperineal resection and node-positive disease were independent prognostic factors of 5-year overall survival, 5-year disease-free survival, and systemic recurrence.
CONCLUSION
NCRT with capecitabine and radical surgery showed favorable long-term oncologic outcomes with benefits of acceptable toxicity and convenience. We suggest that capecitabine can be one of the favorable therapeutic options for NCRT in rectal cancer.
MeSH Terms
Figure
Reference
-
1. Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004; 351:1731–1740.2. Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006; 355:1114–1123.3. Gerard JP, Conroy T, Bonnetain F, Bouche O, Chapet O, Closon-Dejardin MT, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006; 24:4620–4625.4. Rich TA, Shepard RC, Mosley ST. Four decades of continuing innovation with fluorouracil: current and future approaches to fluorouracil chemoradiation therapy. J Clin Oncol. 2004; 22:2214–2232.5. Kim JS, Kim JS, Cho MJ, Song KS, Yoon WH. Preoperative chemoradiation using oral capecitabine in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2002; 54:403–408.6. Hofheinz RD, Wenz F, Post S, Matzdorff A, Laechelt S, Hartmann JT, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, noninferiority, phase 3 trial. Lancet Oncol. 2012; 13:579–588.7. Guillem JG, Chessin DB, Cohen AM, Shia J, Mazumdar M, Enker W, et al. Long-term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Ann Surg. 2005; 241:829–836.8. Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012; 30:1926–1933.9. National Institutes of Health, National Cancer Institute, U.S. Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE) version 4.0. Bethesda (MD): National Institutes of Health, National Cancer Institute, U.S. Department of Health and Human Services;2009.10. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009; 250:187–196.11. Roh MS, Colangelo LH, O'Connell MJ, Yothers G, Deutsch M, Allegra CJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009; 27:5124–5130.12. Lange MM, Martz JE, Ramdeen B, Brooks V, Boachie-Adjei K, van de Velde CJ, et al. Long-term results of rectal cancer surgery with a systematical operative approach. Ann Surg Oncol. 2013; 20:1806–1815.13. Fernandez-Martos C, Nogue M, Cejas P, Moreno-Garcia V, Machancoses AH, Feliu J. The role of capecitabine in locally advanced rectal cancer treatment: an update. Drugs. 2012; 72:1057–1073.14. Kovach JS, Beart RW Jr. Cellular pharmacology of fluorinated pyrimidines in vivo in man. Invest New Drugs. 1989; 7:13–25.15. Ishikawa T, Utoh M, Sawada N, Nishida M, Fukase Y, Sekiguchi F, et al. Tumor selective delivery of 5-fluorouracil by capecitabine, a new oral fluoropyrimidine carbamate, in human cancer xenografts. Biochem Pharmacol. 1998; 55:1091–1097.16. Twelves C, Wong A, Nowacki MP, Abt M, Burris H 3rd, Carrato A, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005; 352:2696–2704.17. Van Cutsem E, Twelves C, Cassidy J, Allman D, Bajetta E, Boyer M, et al. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol. 2001; 19:4097–4106.18. Sawada N, Ishikawa T, Sekiguchi F, Tanaka Y, Ishitsuka H. X-ray irradiation induces thymidine phosphorylase and enhances the efficacy of capecitabine (Xeloda) in human cancer xenografts. Clin Cancer Res. 1999; 5:2948–2953.19. Chan AK, Wong AO, Jenken DA. Preoperative capecitabine and pelvic radiation in locally advanced rectal cancer--is it equivalent to 5-FU infusion plus leucovorin and radiotherapy? Int J Radiat Oncol Biol Phys. 2010; 76:1413–1419.20. Hartley A, Ho KF, McConkey C, Geh JI. Pathological complete response following pre-operative chemoradiotherapy in rectal cancer: analysis of phase II/III trials. Br J Radiol. 2005; 78:934–938.21. Dellas K, Hohler T, Reese T, Wurschmidt F, Engel E, Rodel C, et al. Phase II trial of preoperative radiochemotherapy with concurrent bevacizumab, capecitabine and oxaliplatin in patients with locally advanced rectal cancer. Radiat Oncol. 2013; 8:90.22. Wong SJ, Moughan J, Meropol NJ, Anne PR, Kachnic LA, Rashid A, et al. Efficacy endpoints of radiation therapy group protocol 0247: a randomized, phase 2 study of neoadjuvant radiation therapy plus concurrent capecitabine and irinotecan or capecitabine and oxaliplatin for patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2015; 91:116–123.23. Landry JC, Feng Y, Prabhu RS, Cohen SJ, Staley CA, Whittington R, et al. Phase II Trial of Preoperative Radiation With Concurrent Capecitabine, Oxaliplatin, and Bevacizumab Followed by Surgery and Postoperative 5-Fluorouracil, Leucovorin, Oxaliplatin (FOLFOX), and Bevacizumab in Patients With Locally Advanced Rectal Cancer: 5-Year Clinical Outcomes ECOG-ACRIN Cancer Research Group E3204. Oncologist. 2015; 20:615–616.24. Scheithauer W, McKendrick J, Begbie S, Borner M, Burns WI, Burris HA, et al. Oral capecitabine as an alternative to i.v. 5-fluorouracil-based adjuvant therapy for colon cancer: safety results of a randomized, phase III trial. Ann Oncol. 2003; 14:1735–1743.25. Abushullaih S, Saad ED, Munsell M, Hoff PM. Incidence and severity of hand-foot syndrome in colorectal cancer patients treated with capecitabine: a single-institution experience. Cancer Invest. 2002; 20:3–10.26. Cassidy J, Twelves C, Van Cutsem E, Hoff P, Bajetta E, Boyer M, et al. First-line oral capecitabine therapy in metastatic colorectal cancer: a favorable safety profile compared with intravenous 5-fluorouracil/leucovorin. Ann Oncol. 2002; 13:566–575.27. Twelves CJ, Butts CA, Cassidy J, Conroy T, Braud Fd, Diaz-Rubio E, et al. Capecitabine/oxaliplatin, a safe and active first-line regimen for older patients with metastatic colorectal cancer: post hoc analysis of a large phase II study. Clin Colorectal Cancer. 2005; 5:101–107.28. Law WL, Chu KW. Abdominoperineal resection is associated with poor oncological outcome. Br J Surg. 2004; 91:1493–1499.29. Wang L, Gu GL, Li ZW, Peng YF, Gu J. Abdominoperineal excision following preoperative radiotherapy for rectal cancer: unfavorable prognosis even with negative circumferential resection margin. World J Gastroenterol. 2014; 20:9138–9145.30. Kim NK, Baik SH, Seong JS, Kim H, Roh JK, Lee KY, et al. Oncologic outcomes after neoadjuvant chemoradiation followed by curative resection with tumor-specific mesorectal excision for fixed locally advanced rectal cancer: impact of postirradiated pathologic downstaging on local recurrence and survival. Ann Surg. 2006; 244:1024–1030.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Additional 4-week capecitabine during the resting periods after 6-week neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer: long-term oncologic outcomes
- Long-term oncologic outcomes in pathologic tumor response after neoadjuvant chemoradiation for locally advanced rectal cancer
- Can pretreatment platelet-to-lymphocyte and neutrophil-to-lymphocyte ratios predict long-term oncologic outcomes after preoperative chemoradiation followed by surgery for locally advanced rectal cancer?
- A practical review of watch-and-wait approach in rectal cancer
- Neoadjuvant chemoradiotherapy determines the prognostic impact of anastomotic leakage in advanced rectal cancer