Cancer Res Treat.
2018 Apr;50(2):518-529. 10.4143/crt.2017.005.
Phase II Study of Induction Chemotherapy with Docetaxel, Capecitabine, and Cisplatin Plus Bevacizumab for Initially Unresectable Gastric Cancer with Invasion of Adjacent Organs or Paraaortic Lymph Node Metastasis
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. ykkang@amc.seoul.kr
- 2Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2411140
- DOI: http://doi.org/10.4143/crt.2017.005
Abstract
- PURPOSE
The purpose of this study was to evaluate the efficacy and safety of induction chemotherapy with docetaxel, capecitabine, and cisplatin (DXP) plus bevacizumab (BEV) on initially unresectable locally advanced gastric cancer (LAGC) or paraaortic lymph node (PAN) metastatic gastric cancer (GC).
MATERIALS AND METHODS
Patients with LAGC or unresectable PAN metastatic GC received six induction chemotherapy cycles (60 mg/m2 docetaxel intravenously on day 1, 937.5 mg/m2 capecitabine orally twice daily on days 1-14, 60 mg/m2 cisplatin intravenously on day 1, and 7.5 mg/kg BEV intravenously on day 1 every 3 weeks), followed by conversion surgery. The primary endpoint was R0 resection rate.
RESULTS
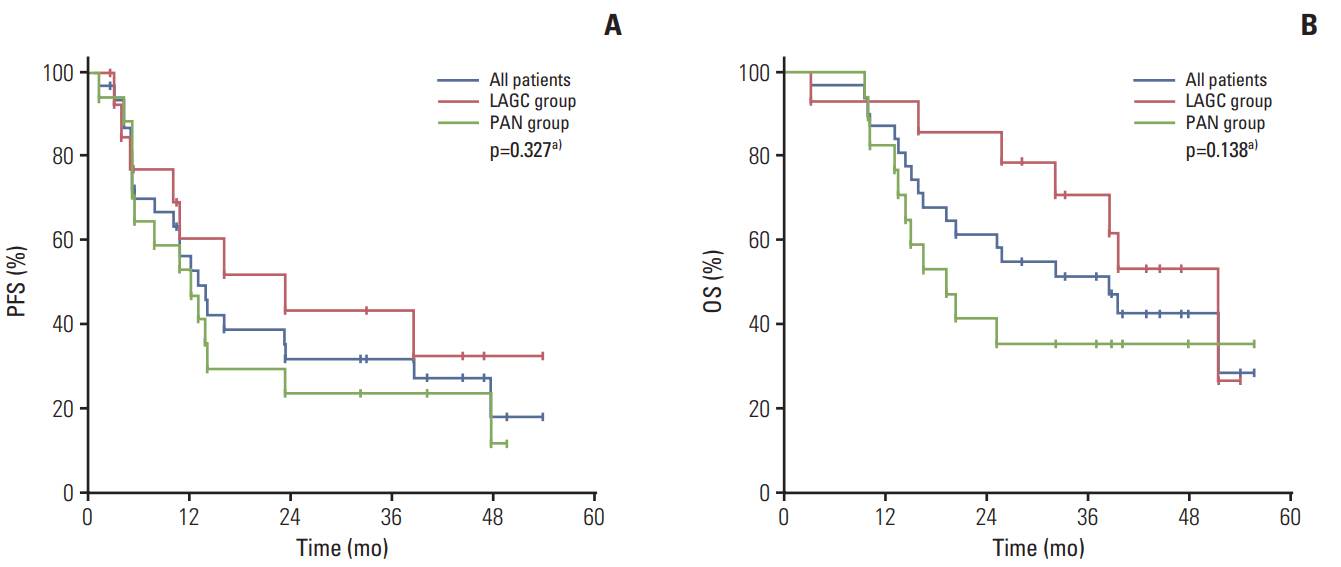
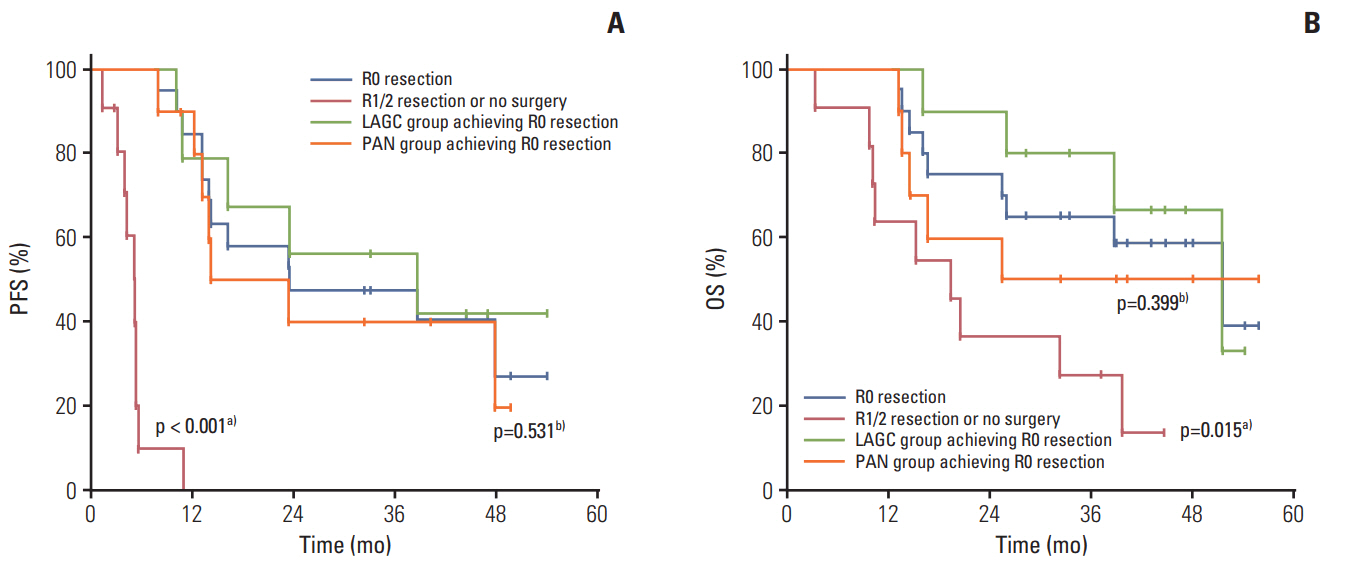
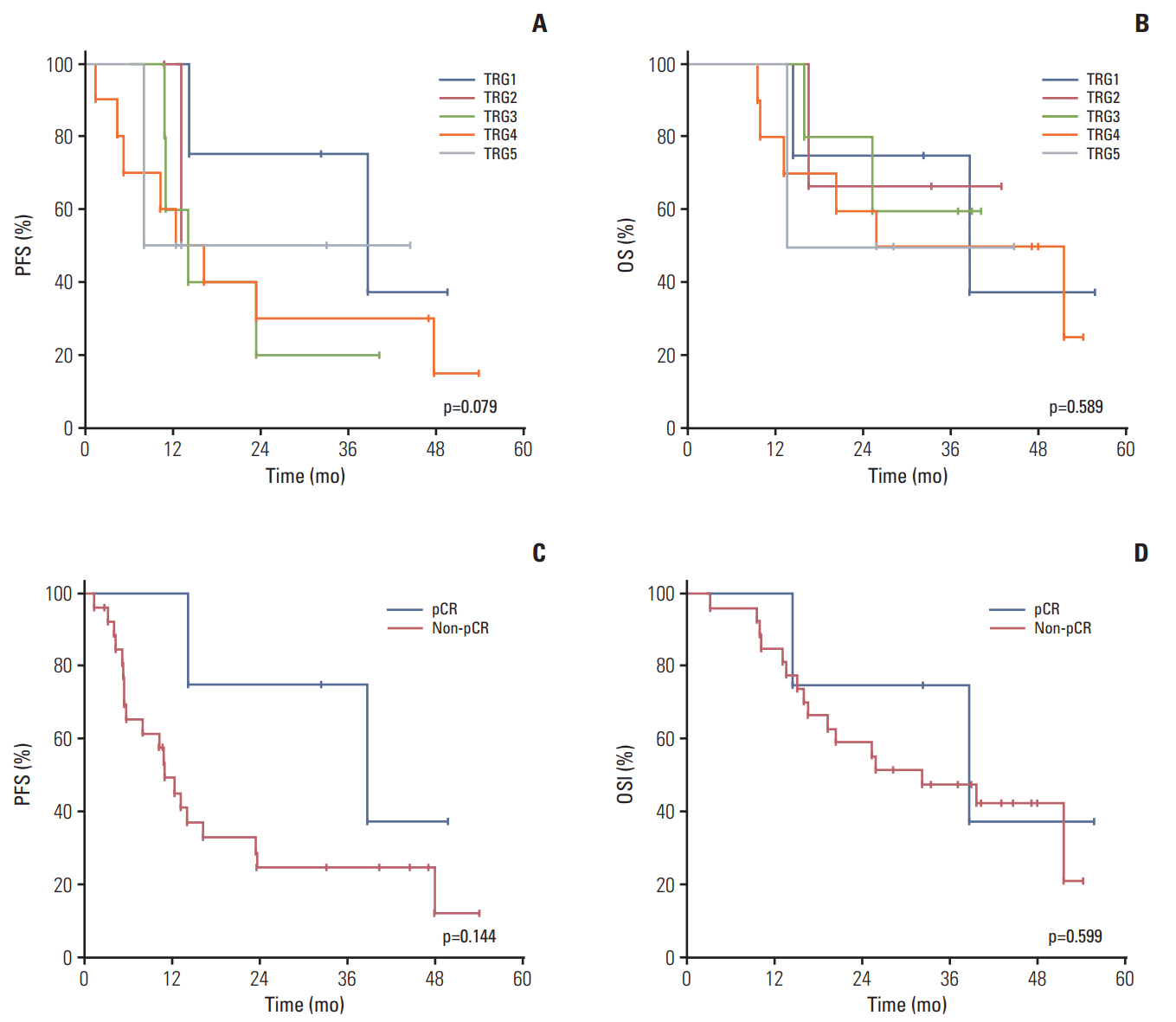
Thirty-one patients with invasion to adjacent organs but without PAN metastasis (n=14, LAGC group) or with PAN metastasis regardless of invasion (n=17, PAN group) were enrolled between July 2010 and December 2014. Twenty-seven patients (87.1%) completed six chemotherapy cycles. The most common grade ≥ 3 toxicities were neutropenia (71%), neutropenia with fever/infection (22.6%/3.2%), and stomatitis (16.1%). The clinical response and R0 resection rates were 64.3% (95% confidence interval [CI], 46.6 to 82.0) and 64.5% (LAGC group, 71.4%; PAN group, 58.8%), respectively. The pathological complete regression rate was 12.9%. After a median follow-up of 44.5 months (range, 39.4 to 49.7 months), the median progression-free survival and overall survival were 13.1 months (95% CI, 8.9 to 17.3) and 38.6 months (95% CI, 22.0 to 55.1), respectively.
CONCLUSION
Induction chemotherapy with DXP+BEV displayed antitumor activities with encouraging R0 resection rate and manageable toxicity profiles on patients with LAGC or PAN metastatic GC.
MeSH Terms
Figure
Reference
-
References
1. Jung KW, Won YJ, Kong HJ, Oh CM, Cho H, Lee DH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015; 47:127–41.
Article2. Koo DH, Ryu MH, Ryoo BY, Seo J, Lee MY, Chang HM, et al. Improving trends in survival of patients who receive chemotherapy for metastatic or recurrent gastric cancer: 12 years of experience at a single institution. Gastric Cancer. 2015; 18:346–53.
Article3. Smalley SR, Benedetti JK, Haller DG, Hundahl SA, Estes NC, Ajani JA, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012; 30:2327–33.
Article4. Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006; 355:11–20.
Article5. Ychou M, Boige V, Pignon JP, Conroy T, Bouche O, Lebreton G, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011; 29:1715–21.
Article6. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007; 357:1810–20.
Article7. Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012; 379:315–21.
Article8. Fukuchi M, Ishiguro T, Ogata K, Suzuki O, Kumagai Y, Ishibashi K, et al. Prognostic role of conversion surgery for unresectable gastric cancer. Ann Surg Oncol. 2015; 22:3618–24.
Article9. Du Y, Yu P. Conversion chemotherapy combined with surgical treatment of unresectable advanced gastric cancer. J Clin Oncol. 2015; 33(3 Suppl):Abstr 189.
Article10. Tsuburaya A, Mizusawa J, Tanaka Y, Fukushima N, Nashimoto A, Sasako M, et al. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with paraaortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg. 2014; 101:653–60.
Article11. Inoue K, Nakane Y, Kogire M, Fujitani K, Kimura Y, Imamura H, et al. Phase II trial of preoperative S-1 plus cisplatin followed by surgery for initially unresectable locally advanced gastric cancer. Eur J Surg Oncol. 2012; 38:143–9.
Article12. Sym SJ, Chang HM, Ryu MH, Lee JL, Kim TW, Yook JH, et al. Neoadjuvant docetaxel, capecitabine and cisplatin (DXP) in patients with unresectable locally advanced or metastatic gastric cancer. Ann Surg Oncol. 2010; 17:1024–32.
Article13. Shah MA, Jhawer M, Ilson DH, Lefkowitz RA, Robinson E, Capanu M, et al. Phase II study of modified docetaxel, cisplatin, and fluorouracil with bevacizumab in patients with metastatic gastroesophageal adenocarcinoma. J Clin Oncol. 2011; 29:868–74.
Article14. Shah MA, Ramanathan RK, Ilson DH, Levnor A, D'Adamo D, O'Reilly E, et al. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol. 2006; 24:5201–6.
Article15. Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011; 29:3968–76.
Article16. Colevas AD, Setser A. The NCI Common Terminology Criteria for Adverse Events (CTCAE) v 3.0 is the new standard for oncology clinical trials. J Clin Oncol. 2004; 22(14 Suppl):Abstr 6098.
Article17. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–16.18. Koh YW, Park YS, Ryu MH, Ryoo BY, Park HJ, Yook JH, et al. Postoperative nodal status and diffuse-type histology are independent prognostic factors in resectable advanced gastric carcinomas after preoperative chemotherapy. Am J Surg Pathol. 2013; 37:1022–9.
Article19. Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma: clinicopathologic correlations. Cancer. 1994; 73:2680–6.
Article20. Becker K, Langer R, Reim D, Novotny A, Meyer zum Buschenfelde C, Engel J, et al. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg. 2011; 253:934–9.21. Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016; 17:309–18.
Article22. Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006; 24:4991–7.
Article23. Kang YK, Ryu MH, Yoo C, Chang HM, Yook JH, Oh ST, et al. Phase I/II study of a combination of docetaxel, capecitabine, and cisplatin (DXP) as first-line chemotherapy in patients with advanced gastric cancer. Cancer Chemother Pharmacol. 2011; 67:1435–43.
Article24. Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004; 350:2335–42.
Article25. Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014; 383:31–9.
Article26. Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014; 15:1224–35.
Article27. Qin S. Phase III study of apatinib in advanced gastric cancer: a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2014; 32(15 Suppl):Abstr 4003.
Article28. Cunningham D, Smyth E, Stenning S, Stevenson L, Robb C, Allum W, et al. Peri-operative chemotherapy ± bevacizumab for resectable gastro-oesophageal adenocarcinoma: results from the UK Medical Research Council randomised ST03 trial (ISRCTN 46020948). Eur J Cancer. 2015; 51 Suppl 3:Abstr 2201.29. Ma J, Yao S, Li XS, Kang HR, Yao FF, Du N. Neoadjuvant therapy of DOF regimen plus bevacizumab can increase surgical resection ratein locally advanced gastric cancer: a randomized, controlled study. Medicine (Baltimore). 2015; 94:e1489.30. Sweeney CJ, Miller KD, Sissons SE, Nozaki S, Heilman DK, Shen J, et al. The antiangiogenic property of docetaxel is synergistic with a recombinant humanized monoclonal antibody against vascular endothelial growth factor or 2-methoxyestradiol but antagonized by endothelial growth factors. Cancer Res. 2001; 61:3369–72.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Curative Resection of Inoperable, Locally Advanced Gastric Cancer after Neoadjuvant Chemotherapy with Taxotere and Cisplatin
- A case of peritoneal metastasis from gastric cancer successfully treated with docetaxel and cisplatin chemotherapy
- Chemotherapy of Advanced Gastric Cancer
- A Multicenter Randomized Phase II Study of Docetaxel vs. Docetaxel Plus Cisplatin vs. Docetaxel Plus S-1 as Second-Line Chemotherapy in Metastatic Gastric Cancer Patients Who Had Progressed after Cisplatin Plus Either S-1 or Capecitabine
- Combination chemotherapy with docetaxel and cisplatin as first-line treatment in advanced gastric cancer: is it a new effective chemotherapy?