Yonsei Med J.
2018 Jun;59(4):470-479. 10.3349/ymj.2018.59.4.470.
Plasma Cell-Free DNA as a Predictive Marker after Radiotherapy for Hepatocellular Carcinoma
- Affiliations
-
- 1Department of Radiation Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea. JSSEONG@yuhs.ac
- KMID: 2410901
- DOI: http://doi.org/10.3349/ymj.2018.59.4.470
Abstract
- PURPOSE
Cell-free DNA (cfDNA) is gaining attention as a novel biomarker for oncologic outcomes. We investigated the clinical significance of cfDNA in hepatocellular carcinoma (HCC) patients treated with radiotherapy (RT).
MATERIALS AND METHODS
Fifty-five patients with HCC who received RT were recruited from two prospective study cohorts: one cohort of 34 patients who underwent conventionally fractionated RT and a second of 21 patients treated with stereotactic body radiation therapy. cfDNA was extracted and quantified.
RESULTS
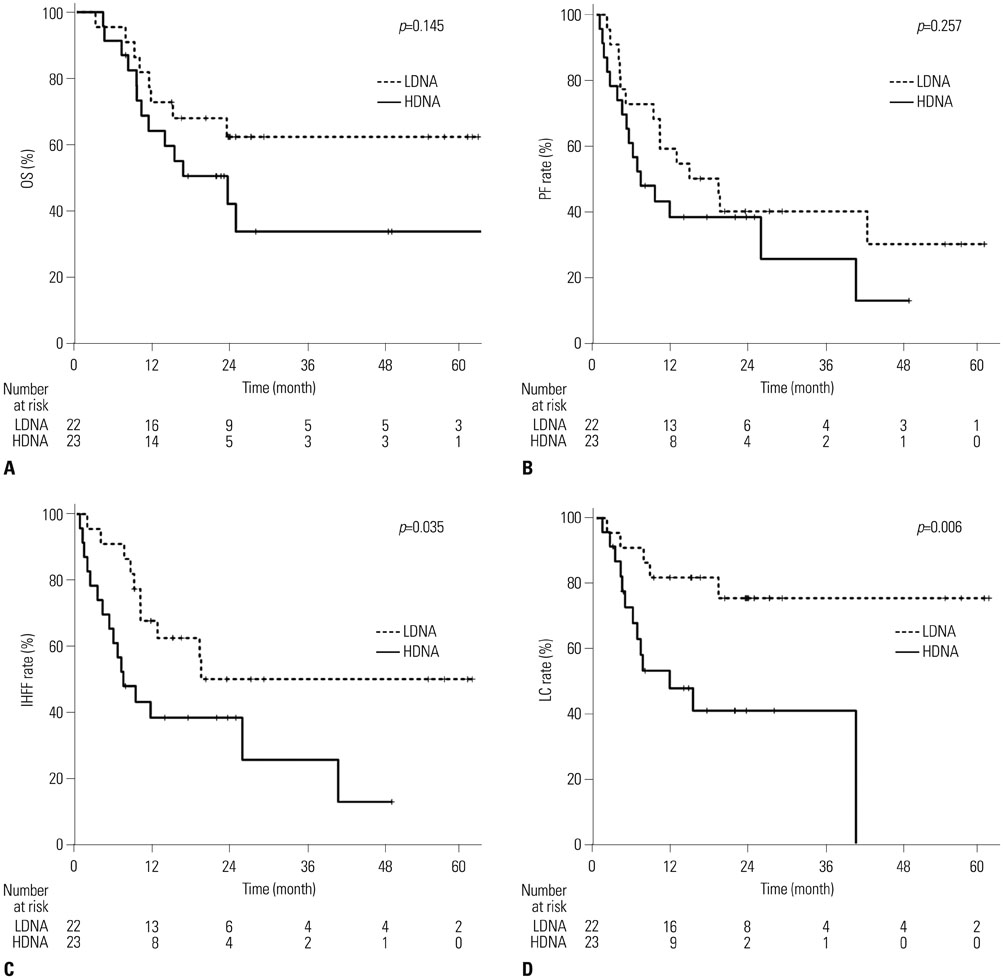
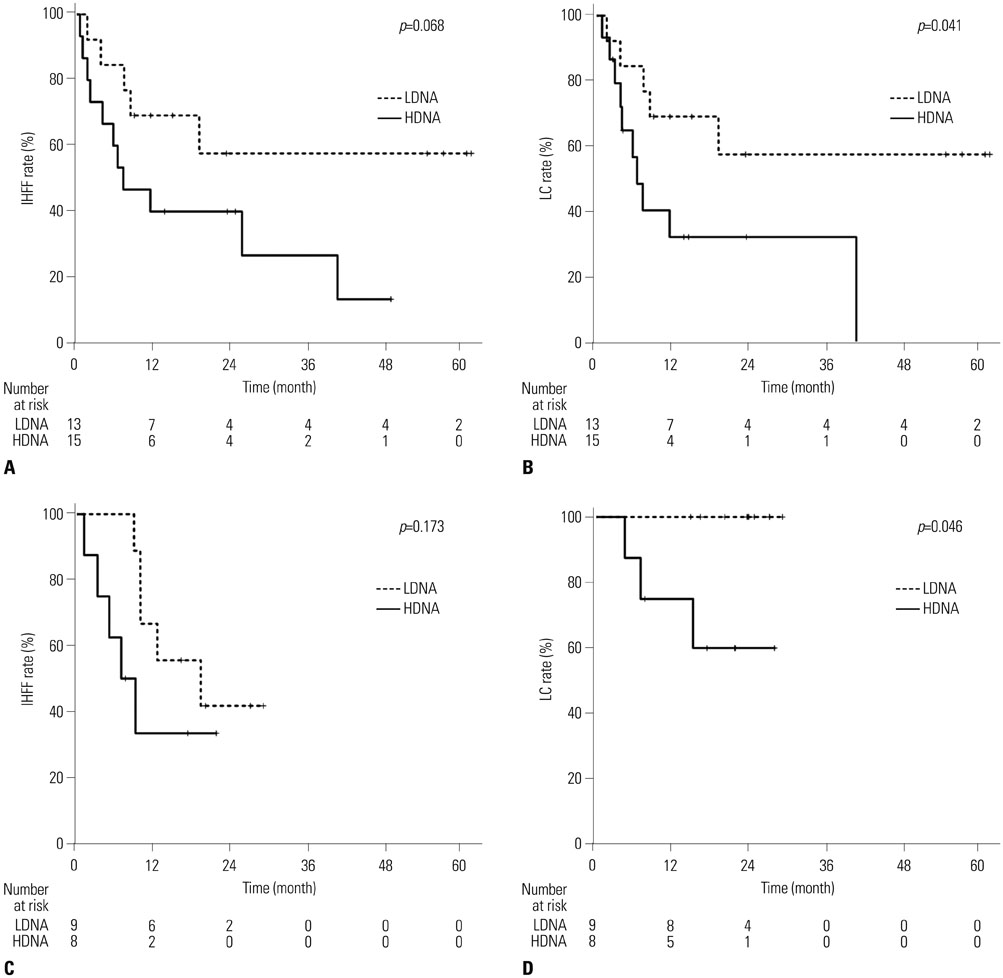
In total, 30% of the patients had multiple tumors, 77% had tumors >2 cm, and 32% had portal vein tumor thrombus. Optimal cut-off values for cfDNA levels (33.65 ng/mL and 37.25 ng/mL, before and after RT) were used to divide patients into low-DNA (LDNA) and high-DNA (HDNA) groups. The pre-RT HDNA group tended to have more advanced disease and larger tumors (p=0.049 and p=0.017, respectively). Tumor response, intrahepatic failure-free rates, and local control (LC) rates were significantly better in the post-RT LDNA group (p=0.017, p=0.035, and p=0.006, respectively).
CONCLUSION
Quantitative analysis of cfDNA was feasible in our cohorts. Post-RT cfDNA levels were negatively correlated with treatment outcomes, indicating the potential for the use of post-RT cfDNA levels as an early predictor of treatment responses and LC after RT for HCC patients.
MeSH Terms
Figure
Reference
-
1. Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer. 2005; 5:845–856.
Article2. Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011; 11:426–437.
Article3. Gorges TM, Pantel K. Circulating tumor cells as therapy-related biomarkers in cancer patients. Cancer Immunol Immunother. 2013; 62:931–939.
Article4. Erpolat OP, Gocun PU, Akmansu M, Karakus E, Akyol G. High expression of nuclear survivin and Aurora B predicts poor overall survival in patients with head and neck squamous cell cancer. Strahlenther Onkol. 2012; 188:248–254.
Article5. Crompton NE, Ozsahin M, Schweizer P, Larsson B, Luetolf UM. Theory and practice of predictive assays in radiation therapy. Strahlenther Onkol. 1997; 173:58–67.
Article6. Baumann M, Krause M, Overgaard J, Debus J, Bentzen SM, Daartz J, et al. Radiation oncology in the era of precision medicine. Nat Rev Cancer. 2016; 16:234–249.
Article7. Shendure J, Ji H. Next-generation DNA sequencing. Nat Biotechnol. 2008; 26:1135–1145.
Article8. Mardis ER. The impact of next-generation sequencing technology on genetics. Trends Genet. 2008; 24:133–141.
Article9. Meldrum C, Doyle MA, Tothill RW. Next-generation sequencing for cancer diagnostics: a practical perspective. Clin Biochem Rev. 2011; 32:177–195.10. Forner A, Vilana R, Ayuso C, Bianchi L, Solė M, Ayuso JR, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008; 47:97–104.
Article11. Bortolin MT, Tedeschi R, Bidoli E, Furlan C, Basaglia G, Minatel E, et al. Cell-free DNA as a prognostic marker in stage I non-small-cell lung cancer patients undergoing stereotactic body radiotherapy. Biomarkers. 2015; 20:422–428.
Article12. Mazurek AM, Rutkowski T, Fiszer-Kierzkowska A, Małusecka E, Składowski K. Assessment of the total cfDNA and HPV16/18 detection in plasma samples of head and neck squamous cell carcinoma patients. Oral Oncol. 2016; 54:36–41.
Article13. Woo SM, Kim MK, Joo J, Yoon KA, Park B, Park SJ, et al. Induction chemotherapy with gemcitabine and cisplatin followed by simultaneous integrated boost-intensity modulated radiotherapy with concurrent gemcitabine for locally advanced unresectable pancreatic cancer: results from a feasibility study. Cancer Res Treat. 2017; 49:1022–1032.
Article14. Kim JW, Seong J, Lee IJ, Woo JY, Han KH. Phase I dose escalation study of helical intensity-modulated radiotherapy-based stereotactic body radiotherapy for hepatocellular carcinoma. Oncotarget. 2016; 7:40756–40766.
Article15. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010; 30:52–60.
Article16. Mandel P, Metais P. Les acides nucleiques du plasma sanguin chez l'homme. C R Seances Soc Biol Fil. 1948; 142:241–243.17. van der Vaart M, Pretorius PJ. Circulating DNA. Its origin and fluctuation. Ann N Y Acad Sci. 2008; 1137:18–26.18. Gormally E, Caboux E, Vineis P, Hainaut P. Circulating free DNA in plasma or serum as biomarker of carcinogenesis: practical aspects and biological significance. Mutat Res. 2007; 635:105–117.
Article19. Cheng C, Omura-Minamisawa M, Kang Y, Hara T, Koike I, Inoue T. Quantification of circulating cell-free DNA in the plasma of cancer patients during radiation therapy. Cancer Sci. 2009; 100:303–309.
Article20. Tang JC, Feng YL, Guo T, Xie AY, Cai XJ. Circulating tumor DNA in hepatocellular carcinoma: trends and challenges. Cell Biosci. 2016; 6:32.
Article21. Iida M, Iizuka N, Sakaida I, Moribe T, Fujita N, Miura T, et al. Relation between serum levels of cell-free DNA and inflammation status in hepatitis C virus-related hepatocellular carcinoma. Oncol Rep. 2008; 20:761–765.
Article22. Kamat AA, Bischoff FZ, Dang D, Baldwin MF, Han LY, Lin YG, et al. Circulating cell-free DNA: a novel biomarker for response to therapy in ovarian carcinoma. Cancer Biol Ther. 2006; 5:1369–1374.
Article23. Schwarzenbach H, Stoehlmacher J, Pantel K, Goekkurt E. Detection and monitoring of cell-free DNA in blood of patients with colorectal cancer. Ann N Y Acad Sci. 2008; 1137:190–196.
Article24. Iizuka N, Sakaida I, Moribe T, Fujita N, Miura T, Stark M, et al. Elevated levels of circulating cell-free DNA in the blood of patients with hepatitis C virus-associated hepatocellular carcinoma. Anticancer Res. 2006; 26:4713–4719.25. Ren N, Ye QH, Qin LX, Zhang BH, Liu YK, Tang ZY. Circulating DNA level is negatively associated with the long-term survival of hepatocellular carcinoma patients. World J Gastroenterol. 2006; 12:3911–3914.
Article26. Liao W, Mao Y, Ge P, Yang H, Xu H, Lu X, et al. Value of quantitative and qualitative analyses of circulating cell-free DNA as diagnostic tools for hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore). 2015; 94:e722.27. Tokuhisa Y, Iizuka N, Sakaida I, Moribe T, Fujita N, Miura T, et al. Circulating cell-free DNA as a predictive marker for distant metastasis of hepatitis C virus-related hepatocellular carcinoma. Br J Cancer. 2007; 97:1399–1403.
Article28. Catarino R, Ferreira MM, Rodrigues H, Coelho A, Nogal A, Sousa A, et al. Quantification of free circulating tumor DNA as a diagnostic marker for breast cancer. DNA Cell Biol. 2008; 27:415–421.
Article29. Zhong XY, Ladewig A, Schmid S, Wight E, Hahn S, Holzgreve W. Elevated level of cell-free plasma DNA is associated with breast cancer. Arch Gynecol Obstet. 2007; 276:327–331.
Article30. Altimari A, Grigioni AD, Benedettini E, Gabusi E, Schiavina R, Martinelli A, et al. Diagnostic role of circulating free plasma DNA detection in patients with localized prostate cancer. Am J Clin Pathol. 2008; 129:756–762.
Article31. Lo YM, Leung SF, Chan LY, Chan AT, Lo KW, Johnson PJ, et al. Kinetics of plasma Epstein-Barr virus DNA during radiation therapy for nasopharyngeal carcinoma. Cancer Res. 2000; 60:2351–2355.32. Wimberger P, Roth C, Pantel K, Kasimir-Bauer S, Kimmig R, Schwarzenbach H. Impact of platinum-based chemotherapy on circulating nucleic acid levels, protease activities in blood and disseminated tumor cells in bone marrow of ovarian cancer patients. Int J Cancer. 2011; 128:2572–2580.
Article33. Tie J, Wang Y, Tomasetti C, Li L, Springer S, Kinde I, et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med. 2016; 8:346ra92.
Article34. Mitsunobu M, Toyosaka A, Oriyama T, Okamoto E, Nakao N. Intrahepatic metastases in hepatocellular carcinoma: the role of the portal vein as an efferent vessel. Clin Exp Metastasis. 1996; 14:520–529.
Article35. Shimada M, Hamatsu T, Yamashita Y, Rikimaru T, Taguchi K, Utsunomiya T, et al. Characteristics of multicentric hepatocellular carcinomas: comparison with intrahepatic metastasis. World J Surg. 2001; 25:991–995.
Article36. Lee JI, Kim JK, Kim DY, Ahn SH, Park JY, Kim SU, et al. Prognosis of hepatocellular carcinoma patients with extrahepatic metastasis and the controllability of intrahepatic lesions. Clin Exp Metastasis. 2014; 31:475–482.
Article37. Furtado LV, Segal JP. Circulating tumor DNA testing for liver cancer. Cell Mol Gastroenterol Hepatol. 2015; 1:458–459.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cell-Free DNA in Oncology: Gearing up for Clinic
- Differences in radiotherapy application according to regional disease characteristics of hepatocellular carcinoma
- Recent developments in radiotherapy for hepatocellular carcinoma
- Novel paradigm in the treatment of hepatocellular carcinoma: Anticipating breakthroughs with particle therapy
- Current status of stereotactic body radiotherapy for the treatment of hepatocellular carcinoma