Korean J Physiol Pharmacol.
2018 May;22(3):343-348. 10.4196/kjpp.2018.22.3.343.
Increased store-operated Ca²⺠entry mediated by GNB5 and STIM1
- Affiliations
-
- 1Department of Oral Biology, BK21 PLUS Project, Yonsei University College of Dentistry, Seoul 03722, Korea. dmshin@yuhs.ac
- KMID: 2410098
- DOI: http://doi.org/10.4196/kjpp.2018.22.3.343
Abstract
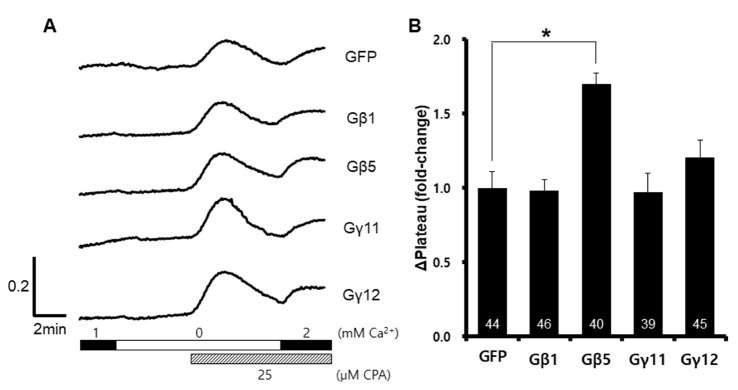
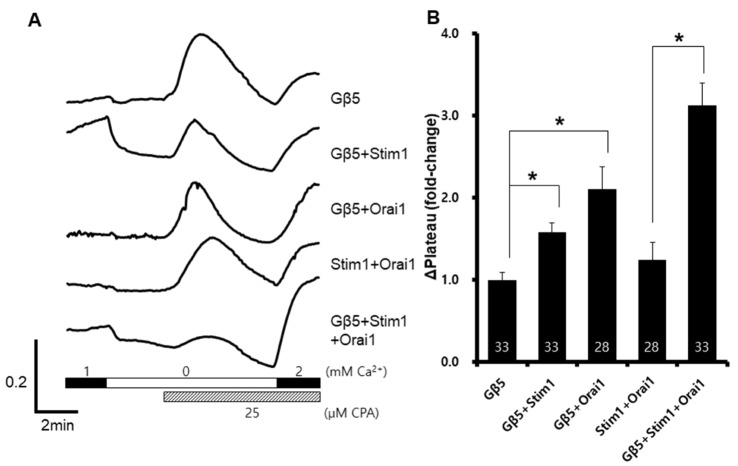
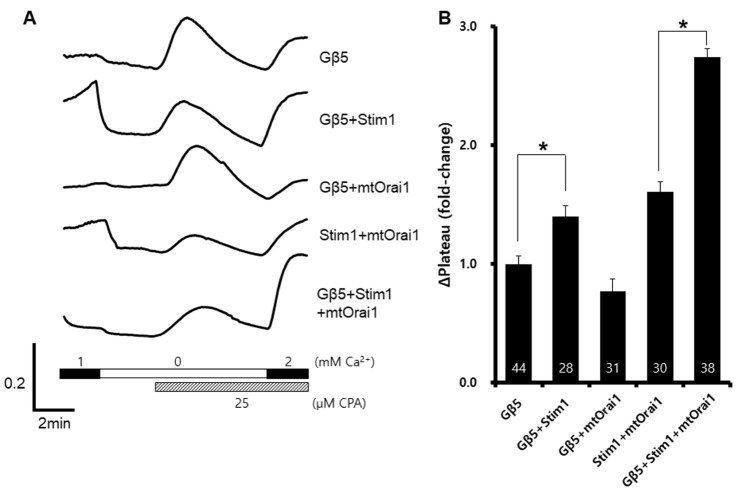
- Recent human genetic studies have shown that Gβ5 is related to various clinical symptoms, such as sinus bradycardia, cognitive disability, and attention deficit hyperactivity disorder. Although the calcium signaling cascade is closely associated with a heterotrimeric G-protein, the function of Gβ5 in calcium signaling and its relevance to clinical symptoms remain unknown. In this study, we investigated the in vitro changes of store-operated calcium entry (SOCE) with exogenous expression of Gβ5. The cells expressing Gβ5 had enhanced SOCE after depletion of calcium ion inside the endoplasmic reticulum. Gβ5 also augmented Stim1- and Orai1-dependent SOCE. An ORAI1 loss-of-function mutant did not show inhibition of Gβ5-induced SOCE, and a STIM1-ERM truncation mutant showed no enhancement of SOCE. These results suggested a novel role of GNB5 and Stim1, and provided insight into the regulatory mechanism of SOCE.
Keyword
MeSH Terms
Figure
Reference
-
1. Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987; 325:321–326. PMID: 2433589.2. Dupré DJ, Robitaille M, Rebois RV, Hébert TE. The role of Gbetagamma subunits in the organization, assembly, and function of GPCR signaling complexes. Annu Rev Pharmacol Toxicol. 2009; 49:31–56. PMID: 18834311.3. Smrcka AV. G protein βγ subunits: central mediators of G protein-coupled receptor signaling. Cell Mol Life Sci. 2008; 65:2191–2214. PMID: 18488142.
Article4. Kisselev O, Ermolaeva M, Gautam N. Efficient interaction with a receptor requires a specific type of prenyl group on the G protein gamma subunit. J Biol Chem. 1995; 270:25356–25358. PMID: 7592699.5. Macrez-Leprêtre N, Kalkbrenner F, Morel JL, Schultz G, Mironneau J. G protein heterotrimer Galpha13beta1gamma3 couples the angiotensin AT1A receptor to increases in cytoplasmic Ca2+ in rat portal vein myocytes. J Biol Chem. 1997; 272:10095–10102. PMID: 9092554.6. Lodder EM, De Nittis P, Koopman CD, Wiszniewski W, Moura de Souza CF, Lahrouchi N, Guex N, Napolioni V, Tessadori F, Beekman L, Nannenberg EA, Boualla L, Blom NA, de Graaff W, Kamermans M, Cocciadiferro D, Malerba N, Mandriani B, Akdemir ZHC, Fish RJ, Eldomery MK, Ratbi I, Wilde AAM, de Boer T, Simonds WF, Neerman-Arbez M, Sutton VR, Kok F, Lupski JR, Reymond A, Bezzina CR, Bakkers J, Merla G. GNB5 mutations cause an autosomal-recessive multisystem syndrome with sinus bradycardia and cognitive disability. Am J Hum Genet. 2016; 99:704–710. PMID: 27523599.
Article7. Shamseldin HE, Masuho I, Alenizi A, Alyamani S, Patil DN, Ibrahim N, Martemyanov KA, Alkuraya FS. GNB5 mutation causes a novel neuropsychiatric disorder featuring attention deficit hyperactivity disorder, severely impaired language development and normal cognition. Genome Biol. 2016; 17:195. PMID: 27677260.
Article8. Xie K, Allen KL, Kourrich S, Colón-Saez J, Thomas MJ, Wickman K, Martemyanov KA. Gbeta5 recruits R7 RGS proteins to GIRK channels to regulate the timing of neuronal inhibitory signaling. Nat Neurosci. 2010; 13:661–663. PMID: 20453851.9. Xie K, Ge S, Collins VE, Haynes CL, Renner KJ, Meisel RL, Lujan R, Martemyanov KA. Gβ5-RGS complexes are gatekeepers of hyper-activity involved in control of multiple neurotransmitter systems. Psychopharmacology (Berl). 2012; 219:823–834. PMID: 21766168.
Article10. Collins HE, Zhu-Mauldin X, Marchase RB, Chatham JC. STIM1/Orai1-mediated SOCE: current perspectives and potential roles in cardiac function and pathology. Am J Physiol Heart Circ Physiol. 2013; 305:H446–H458. PMID: 23792674.
Article11. Kar P, Parekh A. STIM proteins, Orai1 and gene expression. Channels (Austin). 2013; 7:374–378. PMID: 23765192.
Article12. Ruhle B, Trebak M. Emerging roles for native Orai Ca2+ channels in cardiovascular disease. Curr Top Membr. 2013; 71:209–235. PMID: 23890117.13. Shaikh S, Troncoso R, Criollo A, Bravo-Sagua R, García L, Morselli E, Cifuentes M, Quest AF, Hill JA, Lavandero S. Regulation of cardiomyocyte autophagy by calcium. Am J Physiol Endocrinol Metab. 2016; 310:E587–E596. PMID: 26884385.
Article14. Russell VA, Sagvolden T, Johansen EB. Animal models of attention-deficit hyperactivity disorder. Behav Brain Funct. 2005; 1:9. PMID: 16022733.
Article15. Lehohla M, Kellaway L, Russell VA. NMDA receptor function in the prefrontal cortex of a rat model for attention-deficit hyperactivity disorder. Metab Brain Dis. 2004; 19:35–42. PMID: 15214504.
Article16. Lehohla M, Russell V, Kellaway L. NMDA-stimulated Ca2+ uptake into barrel cortex slices of spontaneously hypertensive rats. Metab Brain Dis. 2001; 16:133–141. PMID: 11769326.17. Horn JL, Janicki PK, Franks JJ. Diminished brain synaptic plasma membrane Ca2+-ATPase activity in spontaneously hypertensive rats: association with reduced anesthetic requirements. Life Sci. 1995; 56:PL427–PL432. PMID: 7746091.18. Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, Birnbaumer L. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc Natl Acad Sci U S A. 2008; 105:2895–2900. PMID: 18287061.
Article19. Lee KP, Yuan JP, Hong JH, So I, Worley PF, Muallem S. An endoplasmic reticulum/plasma membrane junction: STIM1/Orai1/TRPCs. FEBS Lett. 2010; 584:2022–2027. PMID: 19944100.
Article20. Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005; 15:1235–1241. PMID: 16005298.21. Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, Icrac and TRPC1 channels. Nat Cell Biol. 2006; 8:1003–1010. PMID: 16906149.22. Hogan PG, Rao A. Store-operated calcium entry: Mechanisms and modulation. Biochem Biophys Res Commun. 2015; 460:40–49. PMID: 25998732.
Article23. Nieto Gutierrez A, McDonald PH. GPCRs: Emerging anti-cancer drug targets. Cell Signal. 2018; 41:65–74. PMID: 28931490.
Article24. Berridge MJ. Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys Acta. 2009; 1793:933–940. PMID: 19010359.
Article25. Myung CS, Garrison JC. Role of C-terminal domains of the G protein beta subunit in the activation of effectors. Proc Natl Acad Sci U S A. 2000; 97:9311–9316. PMID: 10922079.
Article26. Rebres RA, Roach TI, Fraser ID, Philip F, Moon C, Lin KM, Liu J, Santat L, Cheadle L, Ross EM, Simon MI, Seaman WE. Synergistic Ca2+ responses by Gai- and Gaq-coupled G-protein-coupled receptors require a single PLCb isoform that is sensitive to both Gβγ and Gαq. J Biol Chem. 2011; 286:942–951. PMID: 21036901.27. Zeng W, Mak DO, Li Q, Shin DM, Foskett JK, Muallem S. A new mode of Ca2+ signaling by G protein-coupled receptors: gating of IP3 receptor Ca2+ release channels by Gβγ. Curr Biol. 2003; 13:872–876. PMID: 12747838.28. Schindl R, Muik M, Fahrner M, Derler I, Fritsch R, Bergsmann J, Romanin C. Recent progress on STIM1 domains controlling Orai activation. Cell Calcium. 2009; 46:227–232. PMID: 19733393.
Article29. Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009; 11:337–343. PMID: 19182790.
Article30. Sondek J, Bohm A, Lambright DG, Hamm HE, Sigler PB. Crystal structure of a G-protein beta gamma dimer at 2.1A resolution. Nature. 1996; 379:369–374. PMID: 8552196.31. Cao X, Choi S, Maléth JJ, Park S, Ahuja M, Muallem S. The ER/PM microdomain, PI(4,5)P2 and the regulation of STIM1-Orai1 channel function. Cell Calcium. 2015; 58:342–348. PMID: 25843208.
Article32. Jing J, He L, Sun A, Quintana A, Ding Y, Ma G, Tan P, Liang X, Zheng X, Chen L, Shi X, Zhang SL, Zhong L, Huang Y, Dong MQ, Walker CL, Hogan PG, Wang Y, Zhou Y. Proteomic mapping of ER-PM junctions identifies STIMATE as a regulator of Ca2+ influx. Nat Cell Biol. 2015; 17:1339–1347. PMID: 26322679.33. Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006; 312:1220–1223. PMID: 16645049.34. Shin DM, Son A, Park S, Kim MS, Ahuja M, Muallem S. The TRPCs, Orais and STIMs in ER/PM Junctions. Adv Exp Med Biol. 2016; 898:47–66. PMID: 27161224.
Article35. Kim MS, Lee KP, Yang D, Shin DM, Abramowitz J, Kiyonaka S, Birnbaumer L, Mori Y, Muallem S. Genetic and pharmacologic inhibition of the Ca2+ influx channel TRPC3 protects secretory epithelia from Ca2+-dependent toxicity. Gastroenterology. 2011; 140:2107–2115. 2115.e1–2115.e4. PMID: 21354153.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Orai1 and Orai3 in Combination with Stim1 Mediate the Majority of Store-operated Calcium Entry in Astrocytes
- Regulatory mechanisms of the store-operated Ca 2+ entry through Orai1 and STIM1 by an adaptor protein in non-excitable cells
- A focus on extracellular Ca²⺠entry into skeletal muscle
- Store-operated calcium entry in the satellite glial cells of rat sympathetic ganglia
- Regulation of Intracellular Calcium by Endoplasmic Reticulum Proteins in Small Intestinal Interstitial Cells of Cajal