Allergy Asthma Immunol Res.
2018 May;10(3):260-267. 10.4168/aair.2018.10.3.260.
Overexpression of miR-155-5p Inhibits the Proliferation and Migration of IL-13-Induced Human Bronchial Smooth Muscle Cells by Suppressing TGF-β-Activated Kinase 1/MAP3K7-Binding Protein 2
- Affiliations
-
- 1Department of Respiratory Medicine, The Affiliated Changzhou No. 2 People's Hospital of Nanjing Medical University, Changzhou, China. zhangqian_nmu@163.com
- 2Health Science Center, Jiangsu University, Zhenjiang, China.
- KMID: 2409061
- DOI: http://doi.org/10.4168/aair.2018.10.3.260
Abstract
- PURPOSE
Molecular mechanisms leading to asthma is still ill-defined. Though the function of microRNAs (miRNAs) in asthma was previously reported, the involvement of miR-155 in important features of this disease remains unknown. The present study was designed to uncover the probable involvement of miR-155-5p in the proliferation and migration of IL-13-induced human bronchial smooth muscle cells (BSMCs) and the intrinsic regulatory mechanism.
METHODS
The effects of different concentrations of IL-13 on the proliferation and migration of BSMCs as well as the expression of miR-155-5p and its predicted target transforming growth factor (TGF)-β-activated kinase 1/MAP3K7-binding protein 2 (TAB2) were investigated. The effects of miR-155-5p on the proliferation and migration of interleukin (IL)-13-induced BSMCs was determined in vitro using BSMCs transfected with miR-155 mimic/inhibitor and induced by a high concentration of IL-13. The quantitative real-time polymerase chain reaction (qRTPCR) was employed for determining the expression of miR-155-5p and TAB2. Western blotting was applied to analyze the expression of TAB2 at the protein level. Cell proliferation and migration were assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and Transwell assays, respectively.
RESULTS
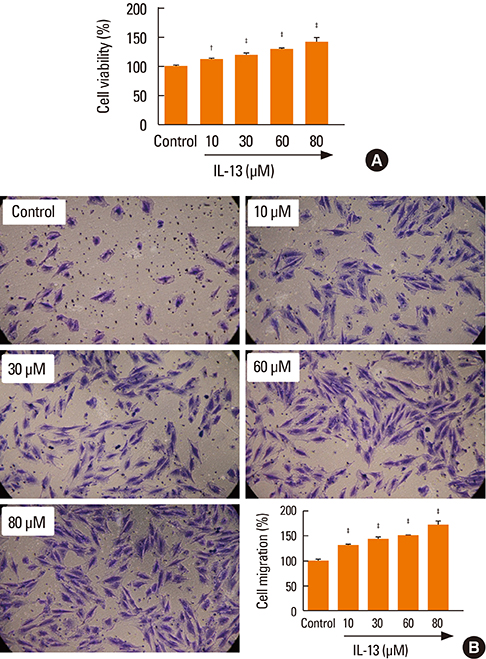
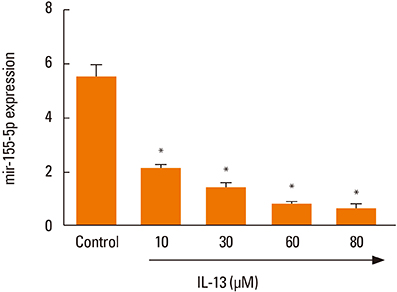
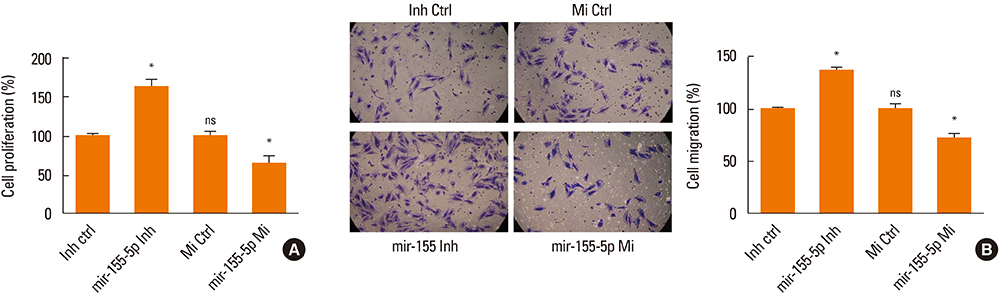
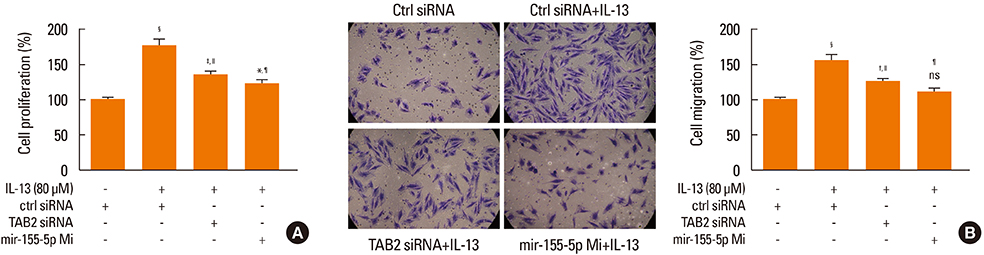
The proliferation and migration of BSMCs were dose-dependently increased with IL-13 treatment. Contrariwise, IL-13 dose-dependently inhibited the expression of miR-155-5p in BSMCs. Mechanistic studies showed that inhibition of miR-155-5p further promoted the stimulatory effects of IL-13, whereas overexpression of miR-155 significantly inhibited these effects. In silico studies and luciferase reporter assays indicated that TAB2 was a negatively regulated miR-155-5p target.
CONCLUSIONS
These results suggested that miR-155-5p-inhibit the IL-13-induced proliferation and migration of BSMCs by targeting TAB2 and that the IL-13/miR-155/TAB2 pathway could serve as a therapeutic target for pulmonary diseases, especially asthma.
Keyword
MeSH Terms
-
Asthma
Blotting, Western
Cell Proliferation
Computer Simulation
Humans*
In Vitro Techniques
Interleukin-13
Interleukins
Luciferases
Lung Diseases
MicroRNAs
Muscle, Smooth*
Myocytes, Smooth Muscle*
Phosphotransferases*
Real-Time Polymerase Chain Reaction
Transforming Growth Factors
Interleukin-13
Interleukins
Luciferases
MicroRNAs
Phosphotransferases
Transforming Growth Factors
Figure
Reference
-
1. Ariel D, Upadhyay D. The role and regulation of microRNAs in asthma. Curr Opin Allergy Clin Immunol. 2012; 12:49–52.
Article2. Ghosh S, Erzurum SC. Nitric oxide metabolism in asthma pathophysiology. Biochim Biophys Acta. 2011; 1810:1008–1016.
Article3. Gras D, Bourdin A, Chanez P, Vachier I. Airway remodeling in asthma: clinical and functional correlates. Med Sci (Paris). 2011; 27:959–965.4. Chung KF. Asthma phenotyping: a necessity for improved therapeutic precision and new targeted therapies. J Intern Med. 2016; 279:192–204.
Article5. Lubret M, Bervar JF, Thumerelle C, Deschildre A, Tillie-Leblond I. Asthma: treatment of exacerbations. Rev Mal Respir. 2012; 29:245–253.6. Moss RB. Treatment options in severe fungal asthma and allergic bronchopulmonary aspergillosis. Eur Respir J. 2014; 43:1487–1500.
Article7. Ricciardolo FL, Blasi F, Centanni S, Rogliani P. Therapeutic novelties of inhaled corticosteroids and bronchodilators in asthma. Pulm Pharmacol Ther. 2015; 33:1–10.
Article8. Dunican EM, Fahy JV. The role of type 2 inflammation in the pathogenesis of asthma exacerbations. Ann Am Thorac Soc. 2015; 12:Suppl 2. S144–S149.9. Hu D. Role of anti-inflammatory cytokines IL-35 and IL-37 in asthma. Inflammation. 2017; 40:697–707.
Article10. Walsh GM. Anti-IL-4/-13 based therapy in asthma. Expert Opin Emerg Drugs. 2015; 20:349–352.
Article11. Albers FC, Price RG, Smith SG, Yancey SW. Mepolizumab efficacy in patients with severe eosinophilic asthma receiving different controller therapies. J Allergy Clin Immunol. 2017; 140:1464–1466.e4.
Article12. Máspero J. Reslizumab in the treatment of inadequately controlled asthma in adults and adolescents with elevated blood eosinophils: clinical trial evidence and future prospects. Ther Adv Respir Dis. 2017; 11:311–325.
Article13. Saha SK, Berry MA, Parker D, Siddiqui S, Morgan A, May R, et al. Increased sputum and bronchial biopsy IL-13 expression in severe asthma. J Allergy Clin Immunol. 2008; 121:685–691.
Article14. Cai F, Hornauer H, Peng K, Schofield CA, Scheerens H, Morimoto AM. Bioanalytical challenges and improved detection of circulating levels of IL-13. Bioanalysis. 2016; 8:323–332.
Article15. Chapmana AM, Malkin DJ, Camacho J, Schiestl RH. IL-13 overexpression in mouse lungs triggers systemic genotoxicity in peripheral blood. Mutat Res. 2014; 769:100–107.16. Ferreira CM, Pereira AT, de Souza RS, Coelho FM, Poole S, Teixeira MM, et al. Role of IL-13 in a model of Strongyloides venezuelensis infection in rats. Microbes Infect. 2010; 12:409–414.
Article17. Brightling CE, Saha S, Hollins F. Interleukin-13: prospects for new treatments. Clin Exp Allergy. 2010; 40:42–49.
Article18. Borowski A, Kuepper M, Horn U, Knupfer U, Zissel G, Hohne K, et al. Interleukin-13 acts as an apoptotic effector on lung epithelial cells and induces pro-fibrotic gene expression in lung fibroblasts. Clin Exp Allergy. 2008; 38:619–628.
Article19. Crapster-Pregont M, Yeo J, Sanchez RL, Kuperman DA. Dendritic cells and alveolar macrophages mediate IL-13-induced airway inflammation and chemokine production. J Allergy Clin Immunol. 2012; 129:1621–1627.e3.
Article20. Firszt R, Francisco D, Church TD, Thomas JM, Ingram JL, Kraft M. Interleukin-13 induces collagen type-1 expression through matrix metalloproteinase-2 and transforming growth factor-beta1 in airway fibroblasts in asthma. Eur Respir J. 2014; 43:464–473.21. Kasaian MT, Miller DK. IL-13 as a therapeutic target for respiratory disease. Biochem Pharmacol. 2008; 76:147–155.
Article22. Mitchell J, Dimov V, Townley RG. IL-13 and the IL-13 receptor as therapeutic targets for asthma and allergic disease. Curr Opin Investig Drugs. 2010; 11:527–534.23. Zhang YY, Zhong M, Zhang MY, Lv K. Expression and clinical significance of miR-155 in peripheral blood CD4(+);T cells of patients with allergic asthma. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2012; 28:540–543.24. Zhou H, Li J, Gao P, Wang Q, Zhang J. miR-155: a novel target in allergic asthma. Int J Mol Sci. 2016; 17:E1773.
Article25. Vock C, Yildirim AO, Wagner C, Schlick S, Lunding LP, Lee CG, et al. Distal airways are protected from goblet cell metaplasia by diminished expression of IL-13 signalling components. Clin Exp Allergy. 2015; 45:1447–1458.
Article26. Parker JC, Thavagnanam S, Skibinski G, Lyons J, Bell J, Heaney LG, et al. Chronic IL9 and IL-13 exposure leads to an altered differentiation of ciliated cells in a well-differentiated paediatric bronchial epithelial cell model. PLoS One. 2013; 8:e61023.
Article27. Qaseem AS, Sonar S, Mahajan L, Madan T, Sorensen GL, Shamji MH, et al. Linking surfactant protein SP-D and IL-13: implications in asthma and allergy. Mol Immunol. 2013; 54:98–107.
Article28. Tomasiak-Łozowska MM, Bodzenta-Łukaszyk A, Tomasiak M, Skiepko R, Zietkowski Z. The role of interleukin 13 and interleukin 5 in asthma. Postepy Hig Med Dosw (Online). 2010; 64:146–155.29. Jakiela B, Gielicz A, Plutecka H, Hubalewska-Mazgaj M, Mastalerz L, Bochenek G, et al. Th2-type cytokine-induced mucus metaplasia decreases susceptibility of human bronchial epithelium to rhinovirus infection. Am J Respir Cell Mol Biol. 2014; 51:229–241.30. Lai HY, Rogers DF. Mucus hypersecretion in asthma: intracellular signalling pathways as targets for pharmacotherapy. Curr Opin Allergy Clin Immunol. 2010; 10:67–76.
Article31. Tanabe T, Fujimoto K, Yasuo M, Tsushima K, Yoshida K, Ise H, et al. Modulation of mucus production by interleukin-13 receptor alpha2 in the human airway epithelium. Clin Exp Allergy. 2008; 38:122–134.32. Cleary RA, Wang R, Wang T, Tang DD. Role of Abl in airway hyperresponsiveness and airway remodeling. Respir Res. 2013; 14:105.
Article33. Jiang H, Xie Y, Abel PW, Toews ML, Townley RG, Casale TB, et al. Targeting phosphoinositide 3-kinase gamma in airway smooth muscle cells to suppress interleukin-13-induced mouse airway hyperresponsiveness. J Pharmacol Exp Ther. 2012; 342:305–311.34. Moynihan B, Tolloczko B, Michoud MC, Tamaoka M, Ferraro P, Martin JG. MAP kinases mediate interleukin-13 effects on calcium signaling in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2008; 295:L171–L177.
Article35. Chiba Y, Nakazawa S, Todoroki M, Shinozaki K, Sakai H, Misawa M. Interleukin-13 augments bronchial smooth muscle contractility with an up-regulation of RhoA protein. Am J Respir Cell Mol Biol. 2009; 40:159–167.
Article36. Espinosa K, Bosse Y, Stankova J, Rola-Pleszczynski M. CysLT1 receptor upregulation by TGF-beta and IL-13 is associated with bronchial smooth muscle cell proliferation in response to LTD4. J Allergy Clin Immunol. 2003; 111:1032–1040.37. Wang Y, Yang F, Xue J, Zhou X, Luo L, Ma Q, et al. Antischistosomiasis liver fibrosis effects of chlorogenic acid through IL-13/miR-21/Smad7 signaling interactions in vivo and in vitro. Antimicrob Agents Chemother. 2017; 61:e01347–e01316.
Article38. Liu Y, Yang K, Shi H, Xu J, Zhang D, Wu Y, et al. MiR-21 modulates human airway smooth muscle cell proliferation and migration in asthma through regulation of PTEN expression. Exp Lung Res. 2015; 41:535–545.
Article39. Comer BS, Camoretti-Mercado B, Kogut PC, Halayko AJ, Solway J, Gerthoffer WT. Cyclooxygenase-2 and microRNA-155 expression are elevated in asthmatic airway smooth muscle cells. Am J Respir Cell Mol Biol. 2015; 52:438–447.
Article40. Greene CM, Gaughan KP. microRNAs in asthma: potential therapeutic targets. Curr Opin Pulm Med. 2013; 19:66–72.41. Chiba Y, Misawa M. MicroRNAs and their therapeutic potential for human diseases: MiR-133a and bronchial smooth muscle hyperresponsiveness in asthma. J Pharmacol Sci. 2010; 114:264–268.
Article42. Chiba Y, Tanabe M, Goto K, Sakai H, Misawa M. Down-regulation of miR-133a contributes to up-regulation of Rhoa in bronchial smooth muscle cells. Am J Respir Crit Care Med. 2009; 180:713–719.
Article43. Malmhäll C, Johansson K, Winkler C, Alawieh S, Ekerljung L, Rådinger M. Altered miR-155 expression in allergic asthmatic airways. Scand J Immunol. 2017; 85:300–307.
Article44. Malmhäll C, Alawieh S, Lu Y, Sjöstrand M, Bossios A, Eldh M, et al. MicroRNA-155 is essential for T(H)2-mediated allergen-induced eosinophilic inflammation in the lung. J Allergy Clin Immunol. 2014; 133:1429–1438. 1438.e1–1438.e7.
Article45. Zech A, Ayata CK, Pankratz F, Meyer A, Baudiss K, Cicko S, et al. MicroRNA-155 modulates P2R signaling and Th2 priming of dendritic cells during allergic airway inflammation in mice. Allergy. 2015; 70:1121–1129.
Article46. Zhang Y, Sun E, Li X, Zhang M, Tang Z, He L, et al. miR-155 contributes to Df1-induced asthma by increasing the proliferative response of Th cells via CTLA-4 downregulation. Cell Immunol. 2017; 314:1–9.
Article47. Xu C, Ren G, Cao G, Chen Q, Shou P, Zheng C, et al. miR-155 regulates immune modulatory properties of mesenchymal stem cells by targeting TAK1-binding protein 2. J Biol Chem. 2013; 288:11074–11079.
Article48. Tan B, Mu R, Chang Y, Wang YB, Wu M, Tu HQ, et al. RNF4 negatively regulates NF-kappaB signaling by down-regulating TAB2. FEBS Lett. 2015; 589:2850–2858.49. Zhu S, Pan W, Song X, Liu Y, Shao X, Tang Y, et al. The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-alpha. Nat Med. 2012; 18:1077–1086.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- MicroRNA-98-5p Inhibits Tumorigenesis of Hepatitis B Virus-Related Hepatocellular Carcinoma by Targeting NF-κB-Inducing Kinase
- Long Non-Coding RNA NORAD Inhibits Breast Cancer Cell Proliferation and Metastasis by Regulating miR-155-5p/ SOCS1 Axis
- MSCs-Derived miR-150-5p-Expressing Exosomes Promote Skin Wound Healing by Activating PI3K/AKT Pathway through PTEN
- In vivo therapeutic success of MicroRNA-155 antagomir in a mouse model of pulmonary fibrosis induced by bleomycin
- Role of NEAT1/MiR-9-5p/SLC26A2 Pathway on Human Airway Smooth Muscle Cell