Nutr Res Pract.
2018 Apr;12(2):129-134. 10.4162/nrp.2018.12.2.129.
Reactive oxygen species-dependent apoptosis induction by water extract of Citrus unshiu peel in MDA-MB-231 human breast carcinoma cells
- Affiliations
-
- 1Open Laboratory for Muscular and Skeletal Disease, and Department of Biochemistry, Dongeui University College of Korean Medicine, 42 San, Yangjungdong, Busan 47227, Korea. choiyh@deu.ac.kr
- 2Anti-Aging Research Center, Dongeui University, Busan 47340, Korea.
- 3Laboratory of Immunobiology, Department of Marine Life Sciences, Jeju National University, Jeju 63243, Korea.
- 4Department of Molecular Biology, College of Natural Sciences, Dongeui University, Busan 47340, Korea.
- KMID: 2407809
- DOI: http://doi.org/10.4162/nrp.2018.12.2.129
Abstract
- BACKGROUND/OBJECTIVES
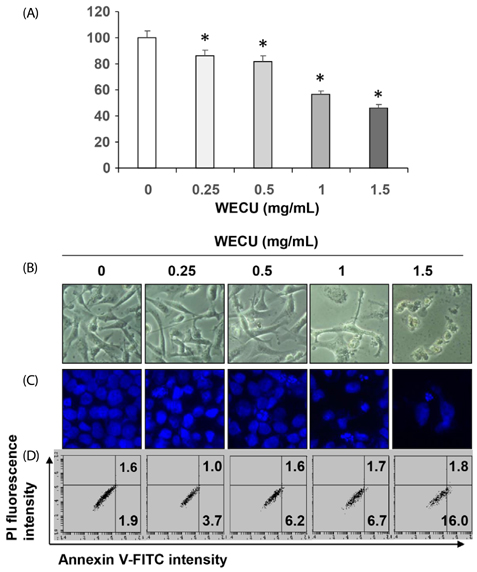
Although several recent studies have reported the anti-cancer effects of extracts or components of Citrus unshiu peel, which has been used for various purposes in traditional medicine, the molecular mechanisms for their effects remain unclear. In the present study, the anti-cancer activity of a water-soluble extract of C. unshiu peel (WECU) in MDA-MB-231 human breast carcinoma cells at the level of apoptosis induction was investigated.
MATERIALS/METHODS
Cytotoxicity was evaluated using the MTT assay. Apoptosis was detected using DAPI staining and flow cytometry analyses. Mitochondrial membrane potential, reactive oxygen species (ROS) assay, caspase activity and Western blotting were used to confirm the basis of apoptosis.
RESULTS
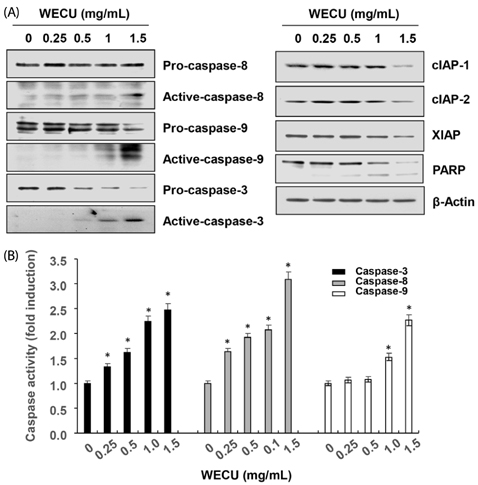
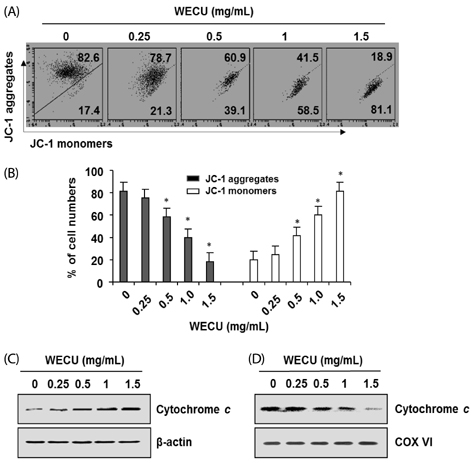
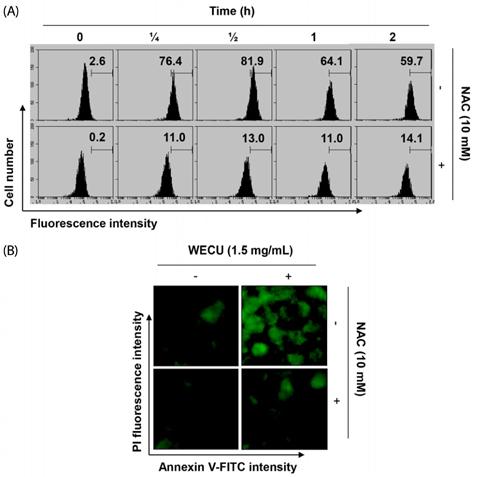
The results indicated that WECU-induced apoptosis was related to the activation of caspase-8, and -9, representative initiator caspases of extrinsic and intrinsic apoptosis pathways, respectively, and caspase-3 accompanied by proteolytic degradation of poly(ADP-ribose) polymerase and down-regulation of the inhibitors of apoptosis protein family members. WECU also increased the pro-apoptotic BAX to anti-apoptotic BCL-2 ratio, loss of mitochondrial membrane potential and cytochrome c release from mitochondria to cytoplasm. Furthermore, WECU provoked the generation of ROS, but the reduction of cell viability and induction of apoptosis by WECU were prevented when ROS production was blocked by antioxidant N-acetyl cysteine.
CONCLUSIONS
These results suggest that WECU suppressed proliferation of MDA-MB-231 cells by activating extrinsic and intrinsic apoptosis pathways in a ROS-dependent manner.
Keyword
MeSH Terms
-
Apoptosis*
Blotting, Western
Breast Neoplasms*
Breast*
Caspase 3
Caspase 8
Caspases, Initiator
Cell Survival
Citrus*
Cysteine
Cytochromes c
Cytoplasm
Down-Regulation
Flow Cytometry
Humans*
Medicine, Traditional
Membrane Potential, Mitochondrial
Mitochondria
Oxygen*
Poly(ADP-ribose) Polymerases
Reactive Oxygen Species
Water*
Caspase 3
Caspase 8
Caspases, Initiator
Cysteine
Cytochromes c
Oxygen
Poly(ADP-ribose) Polymerases
Reactive Oxygen Species
Water
Figure
Reference
-
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016; 66:7–30.
Article2. Niraula S, Ocana A. Mechanism of drug resistance in relation to site of metastasis: meta-analyses of randomized controlled trials in advanced breast cancer according to anticancer strategy. Cancer Treat Rev. 2016; 50:168–174.
Article3. Li C, Yang L, Zhang D, Jiang W. Systematic review and meta-analysis suggest that dietary cholesterol intake increases risk of breast cancer. Nutr Res. 2016; 36:627–635.
Article4. Safarzadeh E, Sandoghchian Shotorbani S, Baradaran B. Herbal medicine as inducers of apoptosis in cancer treatment. Adv Pharm Bull. 2014; 4:421–427.5. Gali-Muhtasib H, Hmadi R, Kareh M, Tohme R, Darwiche N. Cell death mechanisms of plant-derived anticancer drugs: beyond apoptosis. Apoptosis. 2015; 20:1531–1562.
Article6. Bonofiglio D, Giordano C, De Amicis F, Lanzino M, Andò S. Natural products as promising antitumoral agents in breast cancer: mechanisms of action and molecular targets. Mini Rev Med Chem. 2016; 16:596–604.
Article7. Wang CY, Bai XY, Wang CH. Traditional Chinese medicine: a treasured natural resource of anticancer drug research and development. Am J Chin Med. 2014; 42:543–559.
Article8. Ming-Hua C, Bao-Hua Z, Lei Y. Mechanisms of anorexia cancer cachexia syndrome and potential benefits of traditional medicine and natural herbs. Curr Pharm Biotechnol. 2016; 17:1147–1152.
Article9. Min KY, Kim HJ, Lee KA, Kim KT, Paik HD. Antimicrobial activity of acid-hydrolyzed Citrus unshiu peel extract in milk. J Dairy Sci. 2014; 97:1955–1960.
Article10. Park HJ, Jung UJ, Cho SJ, Jung HK, Shim S, Choi MS. Citrus unshiu peel extract ameliorates hyperglycemia and hepatic steatosis by altering inflammation and hepatic glucose- and lipid-regulating enzymes in db/db mice. J Nutr Biochem. 2013; 24:419–427.
Article11. Oh YC, Cho WK, Jeong YH, Im GY, Yang MC, Hwang YH, Ma JY. Anti-inflammatory effect of Citrus unshiu peel in LPS-stimulated RAW 264.7 macrophage cells. Am J Chin Med. 2012; 40:611–629.
Article12. Suzuki M, Sasaki K, Yoshizaki F, Oguchi K, Fujisawa M, Cyong JC. Anti-hepatitis C virus effect of Citrus unshiu peel and its active ingredient nobiletin. Am J Chin Med. 2005; 33:87–94.
Article13. Lee S, Ra J, Song JY, Gwak C, Kwon HJ, Yim SV, Hong SP, Kim J, Lee KH, Cho JJ, Park YS, Park CS, Ahn HJ. Extracts from Citrus unshiu promote immune-mediated inhibition of tumor growth in a murine renal cell carcinoma model. J Ethnopharmacol. 2011; 133:973–979.
Article14. Kim A, Im M, Gu MJ, Ma JY. Citrus unshiu peel extract alleviates cancer-induced weight loss in mice bearing CT-26 adenocarcinoma. Sci Rep. 2016; 6:24214.
Article15. Jin H, Lee WS, Yun JW, Jung JH, Yi SM, Kim HJ, Choi YH, Kim G, Jung JM, Ryu CH, Shin SC, Hong SC. Flavonoids from Citrus unshiu Marc. inhibit cancer cell adhesion to endothelial cells by selective inhibition of VCAM-1. Oncol Rep. 2013; 30:2336–2342.
Article16. Park HR, Park SB, Hong HD, Suh HJ, Shin KS. Structural elucidation of anti-metastatic rhamnogalacturonan II from the pectinase digest of citrus peels (Citrus unshiu). Int J Biol Macromol. 2017; 94:161–169.
Article17. Lee PY, Park BC, Chi SW, Bae KH, Kim S, Cho S, Kang S, Kim JH, Park SG. Histone H4 is cleaved by granzyme A during staurosporine-induced cell death in B-lymphoid Raji cells. BMB Rep. 2016; 49:560–565.
Article18. You MK, Kim HJ, Rhyu J, Kim HA. Pear pomace ethanol extract improves insulin resistance through enhancement of insulin signaling pathway without lipid accumulation. Nutr Res Pract. 2017; 11:198–205.
Article19. Kim HB, Yoo BS. Propolis inhibits UVA-induced apoptosis of human keratinocyte HaCaT cells by scavenging ROS. Toxicol Res. 2016; 32:345–351.
Article20. Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006; 25:4798–4811.
Article21. Nakajima YI, Kuranaga E. Caspase-dependent non-apoptotic processes in development. Cell Death Differ. 2017; 24:1422–1430.
Article22. Decker P, Muller S. Modulating poly (ADP-ribose) polymerase activity: potential for the prevention and therapy of pathogenic situations involving DNA damage and oxidative stress. Curr Pharm Biotechnol. 2002; 3:275–283.
Article23. Hata AN, Engelman JA, Faber AC. The BCL2 family: Key mediators of the apoptotic response to targeted anticancer therapeutics. Cancer Discov. 2015; 5:475–487.
Article24. Kaminskyy VO, Zhivotovsky B. Free radicals in cross talk between autophagy and apoptosis. Antioxid Redox Signal. 2014; 21:86–102.
Article25. Yang Y, Karakhanova S, Hartwig W, D'Haese JG, Philippov PP, Werner J, Bazhin AV. Mitochondria and mitochondrial ROS in cancer: novel targets for anticancer therapy. J Cell Physiol. 2016; 231:2570–2581.
Article26. Kim J, Kim J, Bae JS. ROS homeostasis and metabolism: a critical liaison for cancer therapy. Exp Mol Med. 2016; 48:e269.
Article27. Kim B, Song YS. Mitochondrial dynamics altered by oxidative stress in cancer. Free Radic Res. 2016; 50:1065–1070.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of corosolic acid on apoptosis and angiogenesis in MDA-MB-231 human breast cancer cells
- Delphinidin inhibits cell proliferation and induces apoptosis in MDA-MB-231 human breast cancer cell lines
- Effects of alpha-lipoic acid on cell proliferation and apoptosis in MDA-MB-231 human breast cells
- Effects of Polyamines on TNFalpha- or Tamoxifen-induced Apoptosis in Human Breast Cancer Cells
- Loquat (Eriobotrya japonica) leaf extract inhibits the growth of MDA-MB-231 tumors in nude mouse xenografts and invasion of MDA-MB-231 cells