Nutr Res Pract.
2018 Apr;12(2):110-117. 10.4162/nrp.2018.12.2.110.
Ethanol extract of Allium fistulosum inhibits development of non-alcoholic fatty liver disease
- Affiliations
-
- 1Korea Food Research Institute, 245 Nongsaengmyeong-ro, Jeonbuk 55365, Korea. chkyoung@kfri.re.kr
- 2Department of Food Biotechnology, Korea University of Science & Technology, Daejeon 34113, Korea.
- KMID: 2407807
- DOI: http://doi.org/10.4162/nrp.2018.12.2.110
Abstract
- BACKGROUND/OBJECTIVES
Non-alcoholic fatty liver disease (NAFLD) is a leading cause of chronic liver disease and is closely associated with metabolic syndrome. In the present study, we observed the effect of ethanol extract of Allium fistulosum (EAF) on NAFLD and have suggested the possibility of using EAF as a natural product for application in the development of a treatment for NAFLD.
MATERIALS/METHODS
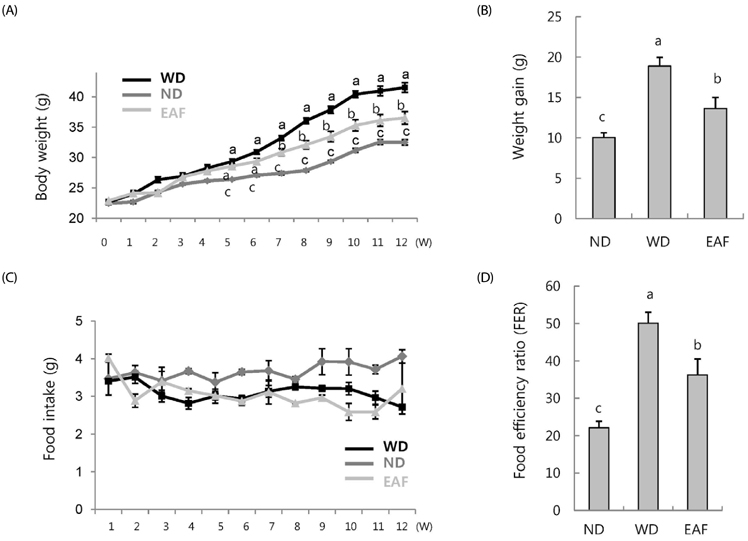
The preventive effect on hepatic lipid accumulation was estimated by using an oleic acid (OA)-induced NAFLD model in vitro and a Western diet (high-fat high-sucrose; WD)-induced obese mouse model. Animals were divided into three groups (n = 7): normal diet group (ND), WD group, and WD plus 1% EAF group.
RESULTS
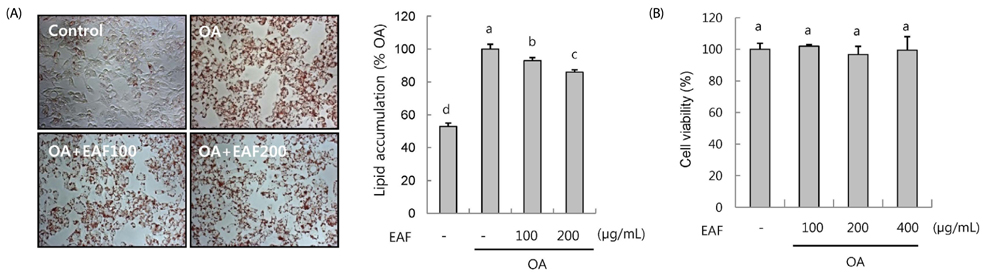
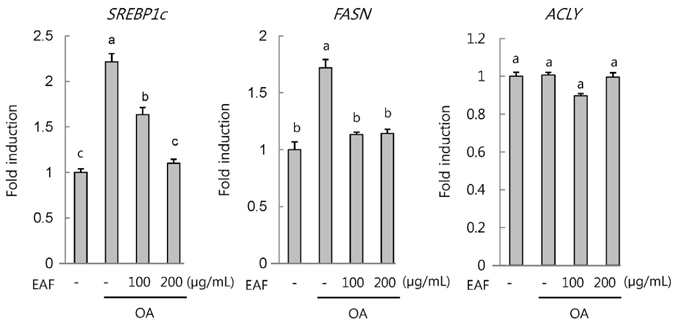
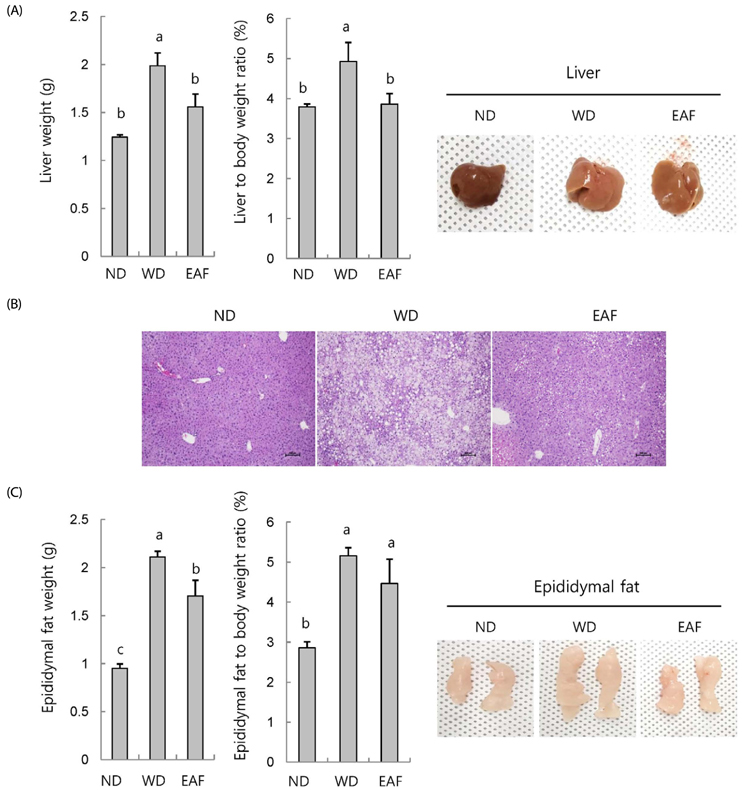
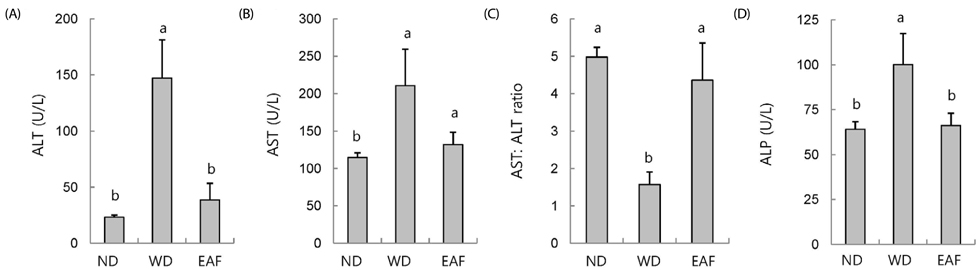
EAF reduced OA-stimulated lipid accumulation in HepG2 cells in the absence of cellular cytotoxicity and significantly blocked transcriptional activation of sterol regulatory element-binding protein 1 and fatty acid synthase genes. Subsequently, we investigated these effects in vivo in mice fed either ND or WD in the presence or absence of EAF supplementation. In comparison to the ND controls, the WD-fed mice exhibited increases in body weight, liver weight, epididymal fat weight, and accumulation of fat in hepatocytes, and these effects were significantly attenuated by EAF supplementation.
CONCLUSIONS
Allium fistulosum attenuates the development of NAFLD, and EAF elicits anti-lipogenic activity in liver. Therefore, EAF represents a promising candidate for use in the development of novel therapeutic drugs or drug combinations for the prevention and treatment of NAFLD.
Keyword
MeSH Terms
-
Allium*
Animals
Body Weight
Diet
Diet, Western
Drug Combinations
Ethanol*
Hep G2 Cells
Hepatocytes
In Vitro Techniques
Lipogenesis
Liver
Liver Diseases
Mice
Mice, Obese
Non-alcoholic Fatty Liver Disease*
Oleic Acid
Sterol Regulatory Element Binding Protein 1
Transcriptional Activation
Drug Combinations
Ethanol
Oleic Acid
Sterol Regulatory Element Binding Protein 1
Figure
Cited by 1 articles
-
Free fatty acid-induced histone acetyltransferase activity accelerates lipid accumulation in HepG2 cells
Sangwon Chung, Jin-Taek Hwang, Jae Ho Park, Hyo-Kyoung Choi
Nutr Res Pract. 2019;13(3):196-204. doi: 10.4162/nrp.2019.13.3.196.
Reference
-
1. Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005; 129:113–121.
Article2. Cohen JC, Hortaon JD, Horbbes HH. Human fatty liver disease: old questions and new insights. Science. 2011; 332:1519–1523.
Article3. Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004; 114:147–152.
Article4. Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004; 10:355–361.
Article5. Lei F, Zhang ZN, Wang W, Xing DM, Xie WD, Su N, Du LJ. Evidence of anti-obesity effects of the pomegranate leaf extract in high-fat diet induced ovese mice. Int J Obes (Lond). 2007; 31:1023–1029.
Article6. Watanabe T, Hata K, Hiwatashi K, Hori K, Suzuki N, Itoh H. Suppression of murine peradipocyte differentiation and reduction of visceral fat accumulation by a Petasites Japonicus ethanol extract in mice fed a high-fat diet. Biosci Biotechnol Biochem. 2010; 74:499–503.
Article7. Willebrords J, Pereira I, Maes M, Yanguas S, Colle I, Bossche B, Silva T, Oliveira C, Andraus W, Alves V, Cogliati B, Vinken M. Strategies, models and biomarkers in experimental non-alcoholic fatty liver disease research. Prog Lipid Res. 2015; 59:106–125.
Article8. Tannapfel A, Denk H, Dienes HP, Langner C, Schirmacher P, Trauner M, Flott-Rahmel B. Histopathological diagnosis of non-alcoholic and alcoholic fatty liver disease. Virchows Arch. 2011; 458:511–523.
Article9. Burt AD, Mutton A, Day CP. Diagnosis and interpretation of steatosis and steatohepatitis. Semin Diagn Pathol. 1998; 15:246–258.10. Tolman KG, Dalpiaz AS. Treatment of non-alcoholic fatty liver disease. Ther Clin Risk Manag. 2007; 3:1153–1163.11. Chalasani N, Guo X, Loomba R, Goodarzi MO, Haritunians T, Kwon S, Cui J, Taylor KD, Wilson L, Cummings OW, Chen YD, Rotter JI. Nonalcoholic Steatohepatitis Clinical Research Network. Genome-wide association study identifies variants associated with histologic features of nonalcoholic fatty liver disease. Gastroenterology. 2010; 139:1567–1576. 1576.e1–1576.e6.
Article12. Anstee QM, Day CP. The genetics NAFLD. Nat Rev Gastroenterol Hepatol. 2013; 10:645–655.13. Ferré P, Foufelle F. Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes Metab. 2010; 12:Suppl 2. 83–92.
Article14. Sung YY, Yoon Y, Kim SJ, Yang WK, Kim HK. Anti-obesity activity of Allium fistulosum L. extract by downregulation of the expression of lipogenic genes in high-fat diet-induced obese mice. Mol Med Rep. 2011; 4:431–435.
Article15. Fenwick GR, Hanley AB. The genius Allium-part 1. Crit Rev Food Sci Nutr. 1985; 22:199–271.16. Chen JH, Chen HI, Wang JS, Tsai SJ, Jen CJ. Effects of Welsh onion extracts on human platelet function in vitro. Life Sci. 2000; 66:1571–1579.
Article17. Yamamoto Y, Aoyama S, Hamaguchi N, Rhi GS. Antioxidative and antihypertensive effects of Welsh onion on rats fed with a high-fat high-sucrose diet. Biosci Biotechnol Biochem. 2005; 69:1311–1317.
Article18. Yamamoto Y, Yasuoka A. Welsh onion attenuates hyperlipidemia in rats fed on high-fat high-sucrose diet. Biosci Biotechnol Biochem. 2010; 74:402–404.
Article19. Beuchat LR. Control of foodborne pathogens and spoilage microorganisms by naturally occurring antimicrobials. In : Wilson CL, Droby S, editors. Microbial Food Contamination. Boca Raton (FL): CRC Press;2001. p. 149–170.20. Pagano G, Pacini G, Musso G, Gambino R, Mecca F, Depetris N, Cassader M, David E, Cavallo-Perin P, Rizzetto M. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: further evidence for an etiologic. Hepatology. 2002; 35:367–372.
Article21. Cui W, Chen SL, Hu KQ. Quantification and mechanisms of oleic acid-induced steatosis in HepG2 cells. Am J Transl Res. 2010; 2:95–104.22. Zhu C, Xie P, Zhao F, Zhang L, An W, Zhan Y. Mechanism of the promotion of steatotic HepG2 cell apoptosis by cholesterol. Int J Clin Exp Pathol. 2014; 7:6807–6813.23. Kang OH, Kin SB, Seo YS, Joung DK, Mun SH, Choi JG, Lee YM, Kang DG, Lee HS, Kwon DY. Curcumin decreases oleic acid-induced lipid accumulation via AMPK phosphorylation in hepatocarcinoma cells. Eur Rev Med Pharmacol Sci. 2013; 17:2578–2586.24. Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002; 109:1125–1131.
Article25. Horton JD, Bashmakov Y, Shimomura I, Shimano H. Regulation o sterol regylatiory element binding proteins in livers of fasted and refed mice. Proc Natl Acad Sci U S A. 1998; 95:5987–5992.
Article26. Horton JD, Shah NA, Warrington JA, Anderson NN, Park SW, Brown MS, Goldstein JL. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci U S A. 2003; 100:12027–12032.
Article27. Shimomura I, Shimano H, Horton JD, Goldstein JL, Brown MS. Differential expression of exons 1a and 1c in mRNAs for sterol regulatory element binding protein-1 in human and mouse organs and cultured cells. J Clin Invest. 1997; 99:838–845.
Article28. Wang BS, Huang GJ, Lu YH, Chang LW. Anti-inflammatory effects of an aqueous extract of Welsh onion green leaves in mice. Food Chem. 2013; 138:751–756.
Article29. Wang BS, Chen JH, Liang YC, Duh PD. Effects of welsh onion on oxidation of low-density lipoprotein and nitric oxide production in macrophage cell line RAW 264.7. Food Chem. 2005; 91:147–155.
Article30. Sharma JN, Al-Omran A, Parvathy SS. Role of nitric oxide in inflammatory diseases. Inflammopharmacology. 2007; 15:252–259.
Article31. Sala A, Folco G. Actual role of prostaglandins in inflammation. Drug Investig. 1991; 3:4–9.
Article32. Aoyama S, Hiraike T, Yamamoto Y. Antioxidant, lipid-lowering and antihypertensive effects of red welsh onion (Allium fistulosum) in spontaneously hypertensive rats. Food Sci Technol Res. 2008; 14:99–103.
Article33. Asrih M, Jornayvaz FR. Inflammation as a potential link between nonalcoholic fatty liver disease and insulin resistance. J Endocrinol. 2013; 218:R25–R36.
Article34. Tilg H, Moschen AR. Evolution on inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010; 52:1836–1846.
Article35. Lima-Cabello E, Garcia-Mediavilla MV, Miguilena-Colina ME, Vargas-Castrillon J, Lozano-Rodriguez T, Femandez-Bermejo M, Olcoz JL, Conzalez-Gallego J, Garcia-Monzon C, Sanchez-Campos S. Enhanced expression on pro-inflammatory mediators and liver X-receptor-regulated lipogenic genes in non-alcoholic fatty liver disease and hepatitis C. Clin Sci (Lond). 2011; 120:239–250.
Article36. Charlton M, Krishnan A, Viker K, Sanderson S, Cazanave S, MeComico A, Masuoko H, Gores G. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am J Physiol Gastrointest Liver Physiol. 2011; 301:G825–G834.
Article37. Kohli R, Kirby M, Xanthakos SA, Softic S, Feldstein AE, Saxena V, Tang PH, Miles L, Miles MV, Balistrere WF, Woods SC, Seeley RJ. High-fructose, medium chain trans fat diet induces liver fibrosis and elevates plasma coenzyme Q9 in a novel murine model of obesity and nonalcoholic steatohepatitis. Hepatology. 2010; 52:934–944.
Article38. Tetri LH, Basaranoglu M, Brunt EM, Yerian LM, Neuschwander-Tetri BA. Severe NAFLD with hepatic necroinflammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent. Am J Physiol Gastrointest Liver Physiol. 2008; 295:G987–G995.
Article39. Machado MV, Michelotti GA, Xie G, Almeida Pereira T, Boursier J, Bohnic B, Guy CD, Diehl AM. Mouse models of diet-induced nonalcoholic steatohepatitis reproduce the heterogeneity of the human disease. PLoS One. 2015; 10:e0127991.
Article40. Almind K, Kahn CR. Genetic determinants of energy expenditure and insulin resistance in diet-induced obesity in mice. Diabetes. 2004; 53:3274–3285.
Article41. Hoevenaars EP, Keijer J, Swarts HJ, Snaas-Alders S, Bekkenkamp-Grovenstein M, van Schothorst EM. Effects of dietary history on energy metabolism and physiological parameters in C57BL/6J mice. Exp Physiol. 2013; 98:1053–1062.
Article42. Bordia A, Verma SK, Vyas AE, Khabya BL, Rathore AS, Bhu N, Dedi HK. Effect of essential oil and garlic on experimental atherosclerosis in rabbits. Atherosclerosis. 1977; 26:379–386.
Article43. Bordia A, Verma SK, Srivastava KC. Effect of garlic (Allium sativum) on blood lipids, blood sugar, fibrinogen and fibrinolytic activity in patients with coronary artery disease. Prostaglandins Leukot Essent Fatty Acids. 1998; 58:257–263.
Article44. Thomson M, Al-Qattan KK, Bordia T, Ali M. Including garlic in the diet may help lower blood glucose, cholesterol, and triglycerides. J Nutr. 2006; 136:800S–802S.
Article45. Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016; 7:27–31.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Activation of Peroxisome Proliferator-Activated Receptor delta Attenuate Alcoholic Liver Disease and Nonalcoholic Fatty Liver Disease in Rats
- Should you advocate for hepatocellular carcinomasurveillance in patients with alcohol-related liverdisease or non-alcoholic fatty liver disease?
- Experimental model and novel therapeutic targets for non-alcoholic fatty liver disease development
- Histologic Risk Factor for Mortality and Development of Severe Liver Disease in Biopsy-proven Non-alcoholic Fatty Liver Disease
- Protective Effects of Ecklonia stolonifera Extract on Ethanol-Induced Fatty Liver in Rats