Korean J Physiol Pharmacol.
2023 Jul;27(4):299-310. 10.4196/kjpp.2023.27.4.299.
Experimental model and novel therapeutic targets for non-alcoholic fatty liver disease development
- Affiliations
-
- 1College of Pharmacy and Institute of Drug Research and Development, Chungnam National University, Daejeon 34134, Korea
- KMID: 2544132
- DOI: http://doi.org/10.4196/kjpp.2023.27.4.299
Abstract
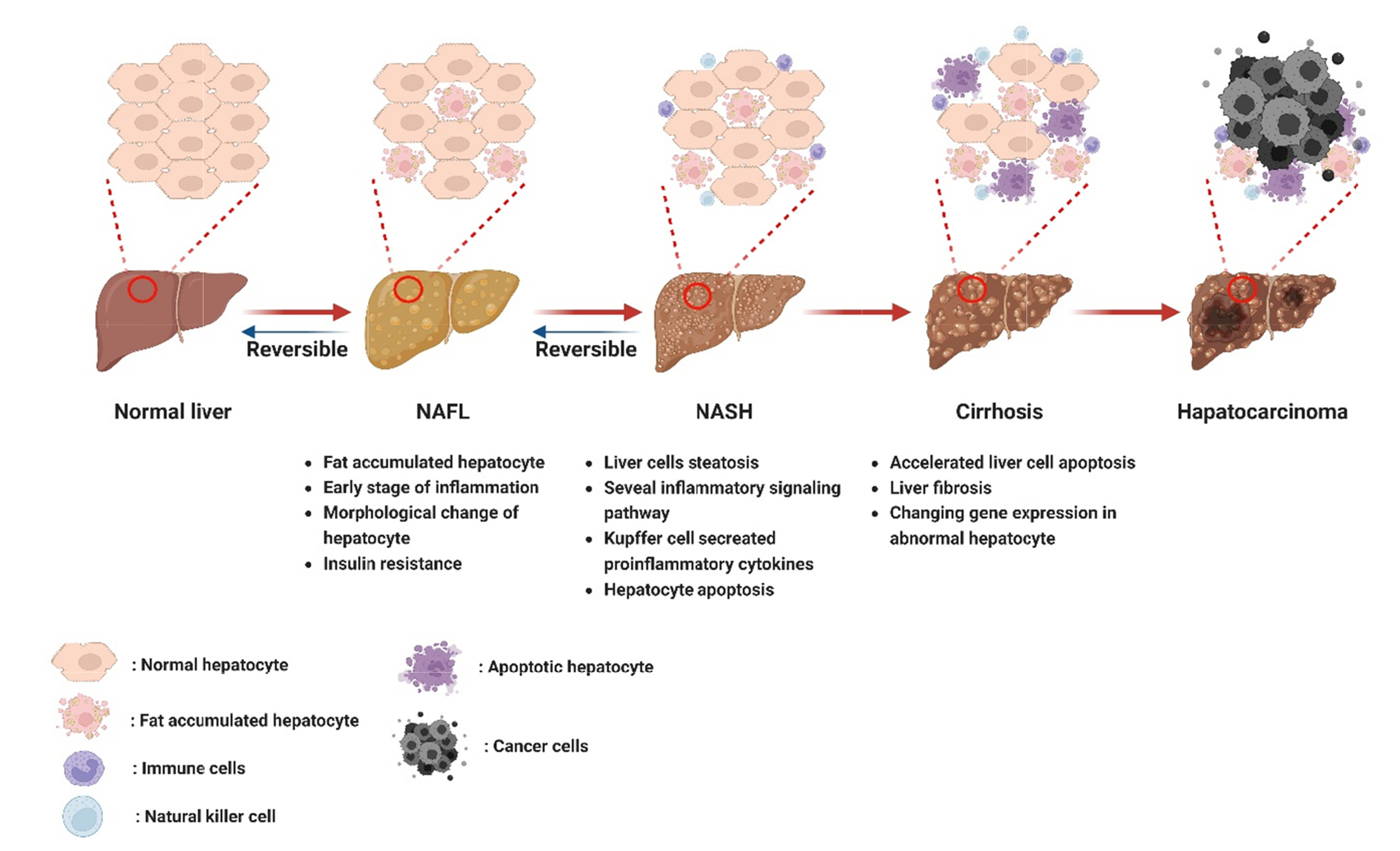
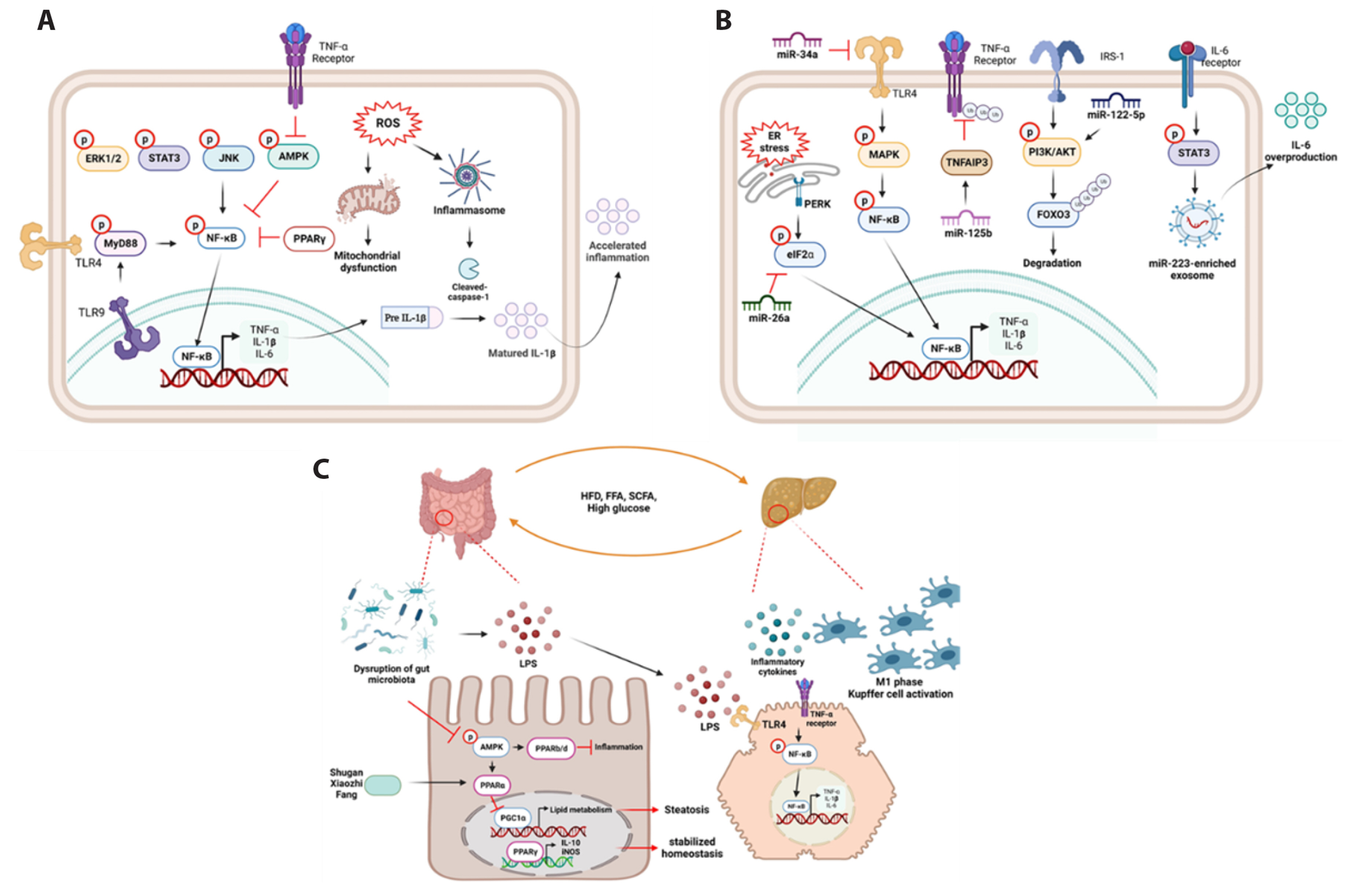
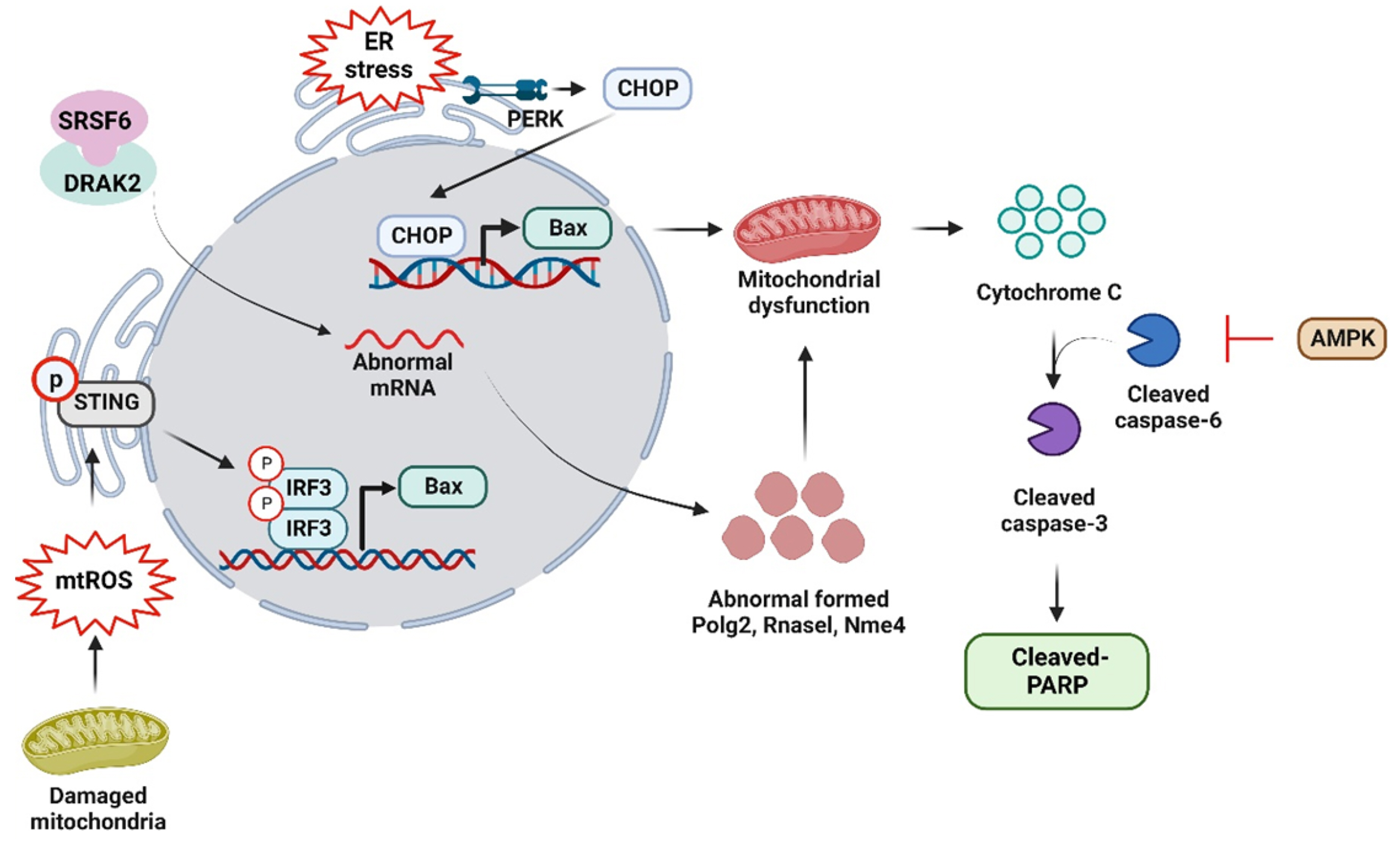
- Non-alcoholic fatty liver disease (NAFLD) is a complex disorder characterized by the accumulation of fat in the liver in the absence of excessive alcohol consumption. It is one of the most common liver diseases worldwide, affecting approximately 25% of the global population. It is closely associated with obesity, type 2 diabetes, and metabolic syndrome. Moreover, NAFLD can progress to non-alcoholic steatohepatitis, which can cause liver cirrhosis, liver failure, and hepatocellular carcinoma. Currently, there are no approved drugs for the treatment of NAFLD. Therefore, the development of effective drugs is essential for NAFLD treatment. In this article, we discuss the experimental models and novel therapeutic targets for NAFLD. Additionally, we propose new strategies for the development of drugs for NAFLD.
Figure
Reference
-
1. Gray ME, Bae S, Ramachandran R, Baldwin N, VanWagner LB, Jacobs DR Jr, Terry JG, Shikany JM. 2022; Dietary patterns and prevalent NAFLD at year 25 from the coronary artery risk development in young adults (CARDIA) study. Nutrients. 14:854. DOI: 10.3390/nu14040854. PMID: 35215504. PMCID: PMC8878386. PMID: 4c852c79ea4c437aac7c4088fabc4aa6.
Article2. Polyzos SA, Kountouras J, Mantzoros CS. 2019; Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism. 92:82–97. DOI: 10.1016/j.metabol.2018.11.014. PMID: 30502373.
Article3. Ter Horst KW, Serlie MJ. 2017; Fructose consumption, lipogenesis, and non-alcoholic fatty liver disease. Nutrients. 9:981. DOI: 10.3390/nu9090981. PMID: 28878197. PMCID: PMC5622741. PMID: 68026d13ef864dd8a97f831d2ab271a0.
Article4. Kumar S, Duan Q, Wu R, Harris EN, Su Q. 2021; Pathophysiological communication between hepatocytes and non-parenchymal cells in liver injury from NAFLD to liver fibrosis. Adv Drug Deliv Rev. 176:113869. DOI: 10.1016/j.addr.2021.113869. PMID: 34280515.
Article5. Tanase DM, Gosav EM, Costea CF, Ciocoiu M, Lacatusu CM, Maranduca MA, Ouatu A, Floria M. 2020; The intricate relationship between type 2 diabetes mellitus (T2DM), insulin resistance (IR), and nonalcoholic fatty liver disease (NAFLD). J Diabetes Res. 2020:3920196. DOI: 10.1155/2020/3920196. PMID: 32832560. PMCID: PMC7424491. PMID: 5534b2bacae74a2dba72bf5cc5ba90ef.
Article6. Ioannou GN. 2021; Epidemiology and risk-stratification of NAFLD-associated HCC. J Hepatol. 75:1476–1484. DOI: 10.1016/j.jhep.2021.08.012. PMID: 34453963.
Article7. Shabalala SC, Dludla PV, Mabasa L, Kappo AP, Basson AK, Pheiffer C, Johnson R. 2020; The effect of adiponectin in the pathogenesis of non-alcoholic fatty liver disease (NAFLD) and the potential role of polyphenols in the modulation of adiponectin signaling. Biomed Pharmacother. 131:110785. DOI: 10.1016/j.biopha.2020.110785. PMID: 33152943.
Article8. Abdel-Bakky MS, Helal GK, El-Sayed EM, Amin E, Alqasoumi A, Alhowail A, Abdelmoti ESS, Saad AS. 2021; Loss of RAR-α and RXR-α and enhanced caspase-3-dependent apoptosis in N-acetyl-p-aminophenol-induced liver injury in mice is tissue factor dependent. Korean J Physiol Pharmacol. 25:385–393. DOI: 10.4196/kjpp.2021.25.5.385. PMID: 34448456. PMCID: PMC8405435.
Article9. Gong G, Zhao R, Zhu Y, Yu J, Wei B, Xu Y, Cui Z, Liang G. 2021; Gastroprotective effect of cirsilineol against hydrochloric acid/ethanol-induced gastric ulcer in rats. Korean J Physiol Pharmacol. 25:403–411. DOI: 10.4196/kjpp.2021.25.5.403. PMID: 34448458. PMCID: PMC8405436.
Article10. Martínez-Chantar ML, Delgado TC, Beraza N. 2021; Revisiting the role of natural killer cells in non-alcoholic fatty liver disease. Front Immunol. 12:640869. DOI: 10.3389/fimmu.2021.640869. PMID: 33679803. PMCID: PMC7930075. PMID: f20ecdd332db4413bf44156c12a70c46.
Article11. Hundertmark J, Krenkel O, Tacke F. 2018; Adapted immune responses of myeloid-derived cells in fatty liver disease. Front Immunol. 9:2418. DOI: 10.3389/fimmu.2018.02418. PMID: 30405618. PMCID: PMC6200865. PMID: a7dbc75d5dd54e3b85c2fdbf219d55a7.
Article12. Puengel T, Liu H, Guillot A, Heymann F, Tacke F, Peiseler M. 2022; Nuclear receptors linking metabolism, inflammation, and fibrosis in nonalcoholic fatty liver disease. Int J Mol Sci. 23:2668. DOI: 10.3390/ijms23052668. PMID: 35269812. PMCID: PMC8910763. PMID: 3bd012ba53de463bb8d3570ead664810.
Article13. Peng C, Stewart AG, Woodman OL, Ritchie RH, Qin CX. 2020; Non-alcoholic steatohepatitis: a review of its mechanism, models and medical treatments. Front Pharmacol. 11:603926. DOI: 10.3389/fphar.2020.603926. PMID: 33343375. PMCID: PMC7745178. PMID: 2dc08ed0b09742598ac9467466fe05ab.
Article14. Márquez-Quiroga LV, Arellanes-Robledo J, Vásquez-Garzón VR, Villa-Treviño S, Muriel P. 2022; Models of nonalcoholic steatohepatitis potentiated by chemical inducers leading to hepatocellular carcinoma. Biochem Pharmacol. 195:114845. DOI: 10.1016/j.bcp.2021.114845. PMID: 34801522.
Article15. Feng M, Liu F, Xing J, Zhong Y, Zhou X. 2021; Anemarrhena saponins attenuate insulin resistance in rats with high-fat diet-induced obesity via the IRS-1/PI3K/AKT pathway. J Ethnopharmacol. 277:114251. DOI: 10.1016/j.jep.2021.114251. PMID: 34052350.
Article16. Marengo A, Rosso C, Bugianesi E. 2016; Liver cancer: connections with obesity, fatty liver, and cirrhosis. Annu Rev Med. 67:103–117. DOI: 10.1146/annurev-med-090514-013832. PMID: 26473416.
Article17. Chyau CC, Wang HF, Zhang WJ, Chen CC, Huang SH, Chang CC, Peng RY. 2020; Antrodan alleviates high-fat and high-fructose diet-induced fatty liver disease in C57BL/6 mice model via AMPK/Sirt1/SREBP-1c/PPARγ pathway. Int J Mol Sci. 21:360. DOI: 10.3390/ijms21010360. PMID: 31935815. PMCID: PMC6981486. PMID: 4f4399ffc2aa42f9af8d7a805e316974.
Article18. Zheng T, Yang X, Li W, Wang Q, Chen L, Wu D, Bian F, Xing S, Jin S. 2018; Salidroside attenuates high-fat diet-induced nonalcoholic fatty liver disease via AMPK-dependent TXNIP/NLRP3 pathway. Oxid Med Cell Longev. 2018:8597897. DOI: 10.1155/2018/8597897. PMID: 30140371. PMCID: PMC6081551.
Article19. Softic S, Stanhope KL, Boucher J, Divanovic S, Lanaspa MA, Johnson RJ, Kahn CR. 2020; Fructose and hepatic insulin resistance. Crit Rev Clin Lab Sci. 57:308–322. DOI: 10.1080/10408363.2019.1711360. PMID: 31935149. PMCID: PMC7774304.
Article20. Tsuchida T, Lee YA, Fujiwara N, Ybanez M, Allen B, Martins S, Fiel MI, Goossens N, Chou HI, Hoshida Y, Friedman SL. 2018; A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer. J Hepatol. 69:385–395. Erratum in: J Hepatol. 2018;69:988. DOI: 10.1016/j.jhep.2018.07.010. PMID: 30082074.
Article21. Im YR, Hunter H, de Gracia Hahn D, Duret A, Cheah Q, Dong J, Fairey M, Hjalmarsson C, Li A, Lim HK, McKeown L, Mitrofan CG, Rao R, Utukuri M, Rowe IA, Mann JP. 2021; A systematic review of animal models of NAFLD finds high-fat, high-fructose diets most closely resemble human NAFLD. Hepatology. 74:1884–1901. DOI: 10.1002/hep.31897. PMID: 33973269.
Article22. Segal-Salto M, Barashi N, Katav A, Edelshtein V, Aharon A, Hashmueli S, George J, Maor Y, Pinzani M, Haberman D, Hall A, Friedman S, Mor A. 2020; A blocking monoclonal antibody to CCL24 alleviates liver fibrosis and inflammation in experimental models of liver damage. JHEP Rep. 2:100064. DOI: 10.1016/j.jhepr.2019.100064. PMID: 32039405. PMCID: PMC7005554.
Article23. Parlati L, Régnier M, Guillou H, Postic C. 2021; New targets for NAFLD. JHEP Rep. 3:100346. DOI: 10.1016/j.jhepr.2021.100346. PMID: 34667947. PMCID: PMC8507191.
Article24. Li X, Wang TX, Huang X, Li Y, Sun T, Zang S, Guan KL, Xiong Y, Liu J, Yuan HX. 2020; Targeting ferroptosis alleviates methionine-choline deficient (MCD)-diet induced NASH by suppressing liver lipotoxicity. Liver Int. 40:1378–1394. DOI: 10.1111/liv.14428. PMID: 32145145.
Article25. Stephenson K, Kennedy L, Hargrove L, Demieville J, Thomson J, Alpini G, Francis H. 2018; Updates on dietary models of nonalcoholic fatty liver disease: current studies and insights. Gene Expr. 18:5–17. DOI: 10.3727/105221617X15093707969658. PMID: 29096730. PMCID: PMC5860971.
Article26. Deng M, Qu F, Chen L, Liu C, Zhang M, Ren F, Guo H, Zhang H, Ge S, Wu C, Zhao L. 2020; SCFAs alleviated steatosis and inflammation in mice with NASH induced by MCD. J Endocrinol. 245:425–437. DOI: 10.1530/JOE-20-0018. PMID: 32302970.
Article27. Veskovic M, Mladenovic D, Milenkovic M, Tosic J, Borozan S, Gopcevic K, Labudovic-Borovic M, Dragutinovic V, Vucevic D, Jorgacevic B, Isakovic A, Trajkovic V, Radosavljevic T. 2019; Betaine modulates oxidative stress, inflammation, apoptosis, autophagy, and Akt/mTOR signaling in methionine-choline deficiency-induced fatty liver disease. Eur J Pharmacol. 848:39–48. DOI: 10.1016/j.ejphar.2019.01.043. PMID: 30689995.
Article28. Zhao W, He C, Jiang J, Zhao Z, Yuan H, Wang F, Shen B. 2022; The role of discoid domain receptor 1 on renal tubular epithelial pyroptosis in diabetic nephropathy. Korean J Physiol Pharmacol. 26:427–438. DOI: 10.4196/kjpp.2022.26.6.427. PMID: 36302618. PMCID: PMC9614395.
Article29. Morishita A, Tadokoro T, Fujihara S, Iwama H, Oura K, Fujita K, Tani J, Takuma K, Nakahara M, Shi T, Haba R, Okano K, Nishiyama A, Ono M, Himoto T, Masaki T. 2022; Ipragliflozin attenuates non-alcoholic steatohepatitis development in an animal model. PLoS One. 17:e0261310. DOI: 10.1371/journal.pone.0261310. PMID: 35192632. PMCID: PMC8863244. PMID: bf48577133404d54ac87805d0b6d9c74.
Article30. Yoshimine Y, Uto H, Kumagai K, Mawatari S, Arima S, Ibusuki R, Mera K, Nosaki T, Kanmura S, Numata M, Tamai T, Moriuchi A, Tsubouchi H, Ido A. 2015; Hepatic expression of the Sptlc3 subunit of serine palmitoyltransferase is associated with the development of hepatocellular carcinoma in a mouse model of nonalcoholic steatohepatitis. Oncol Rep. 33:1657–1666. DOI: 10.3892/or.2015.3745. PMID: 25607821.
Article31. Jin Y, Nguyen TLL, Myung CS, Heo KS. 2022; Ginsenoside Rh1 protects human endothelial cells against lipopolysaccharide-induced inflammatory injury through inhibiting TLR2/4-mediated STAT3, NF-κB, and ER stress signaling pathways. Life Sci. 309:120973. DOI: 10.1016/j.lfs.2022.120973. PMID: 36150463.
Article32. Pawlak M, Lefebvre P, Staels B. 2015; Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 62:720–733. DOI: 10.1016/j.jhep.2014.10.039. PMID: 25450203.
Article33. Van Nguyen D, Nguyen TLL, Jin Y, Kim L, Myung CS, Heo KS. 2022; 6'-Sialylactose abolished lipopolysaccharide-induced inflammation and hyper-permeability in endothelial cells. Arch Pharm Res. 45:836–848. DOI: 10.1007/s12272-022-01415-0. PMID: 36401777.
Article34. Chen Z, Yu R, Xiong Y, Du F, Zhu S. 2017; A vicious circle between insulin resistance and inflammation in nonalcoholic fatty liver disease. Lipids Health Dis. 16:203. Erratum in: Lipids Health Dis. 2018; 17:33. DOI: 10.1186/s12944-017-0572-9. PMID: 29037210. PMCID: PMC5644081. PMID: affc31d5c7b746ebbc0d17caf6f1e363.
Article35. Yan T, Wang H, Cao L, Wang Q, Takahashi S, Yagai T, Li G, Krausz KW, Wang G, Gonzalez FJ, Hao H. 2018; Glycyrrhizin alleviates nonalcoholic steatohepatitis via modulating bile acids and meta-inflammation. Drug Metab Dispos. 46:1310–1319. DOI: 10.1124/dmd.118.082008. PMID: 29959134. PMCID: PMC6081736.
Article36. Li CX, Gao JG, Wan XY, Chen Y, Xu CF, Feng ZM, Zeng H, Lin YM, Ma H, Xu P, Yu CH, Li YM. 2019; Allyl isothiocyanate ameliorates lipid accumulation and inflammation in nonalcoholic fatty liver disease via the Sirt1/AMPK and NF-κB signaling pathways. World J Gastroenterol. 25:5120–5133. DOI: 10.3748/wjg.v25.i34.5120. PMID: 31558861. PMCID: PMC6747284.
Article37. Diniz TA, de Lima Junior EA, Teixeira AA, Biondo LA, da Rocha LAF, Valadão IC, Silveira LS, Cabral-Santos C, de Souza CO, Rosa Neto JC. 2021; Aerobic training improves NAFLD markers and insulin resistance through AMPK-PPAR-α signaling in obese mice. Life Sci. 266:118868. DOI: 10.1016/j.lfs.2020.118868. PMID: 33310034.
Article38. Xiao Q, Zhang S, Yang C, Du R, Zhao J, Li J, Xu Y, Qin Y, Gao Y, Huang W. 2019; Ginsenoside Rg1 ameliorates palmitic acid-induced hepatic steatosis and inflammation in HepG2 cells via the AMPK/NF-κB pathway. Int J Endocrinol. 2019:7514802. DOI: 10.1155/2019/7514802. PMID: 31467529. PMCID: PMC6699274. PMID: 81c42df073804a37833110c45bb3fe67.39. Nguyen TLL, Huynh DTN, Jin Y, Jeon H, Heo KS. 2021; Protective effects of ginsenoside-Rg2 and -Rh1 on liver function through inhibiting TAK1 and STAT3-mediated inflammatory activity and Nrf2/ARE-mediated antioxidant signaling pathway. Arch Pharm Res. 44:241–252. DOI: 10.1007/s12272-020-01304-4. PMID: 33537886.
Article40. Zhu W, Sahar NE, Javaid HMA, Pak ES, Liang G, Wang Y, Ha H, Huh JY. 2021; Exercise-induced irisin decreases inflammation and improves NAFLD by competitive binding with MD2. Cells. 10:3306. DOI: 10.3390/cells10123306. PMID: 34943814. PMCID: PMC8699279. PMID: 72948fe9874b44a18ece0156762b6e01.
Article41. Bai J, Liu F. 2019; The cGAS-cGAMP-STING pathway: a molecular link between immunity and metabolism. Diabetes. 68:1099–1108. DOI: 10.2337/dbi18-0052. PMID: 31109939. PMCID: PMC6610018.
Article42. Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L. 2017; Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 9:7204–7218. DOI: 10.18632/oncotarget.23208. PMID: 29467962. PMCID: PMC5805548.
Article43. Jin Y, Tangchang W, Kwon OS, Lee JY, Heo KS, Son HY. 2023; Ginsenoside Rh1 ameliorates the asthma and allergic inflammation via inhibiting Akt, MAPK, and NF-κB signaling pathways in vitro and in vivo. Life Sci. 321:121607. DOI: 10.1016/j.lfs.2023.121607. PMID: 36958436.
Article44. Mohammed S, Nicklas EH, Thadathil N, Selvarani R, Royce GH, Kinter M, Richardson A, Deepa SS. 2021; Role of necroptosis in chronic hepatic inflammation and fibrosis in a mouse model of increased oxidative stress. Free Radic Biol Med. 164:315–328. DOI: 10.1016/j.freeradbiomed.2020.12.449. PMID: 33429022. PMCID: PMC8845573.
Article45. Gaul S, Leszczynska A, Alegre F, Kaufmann B, Johnson CD, Adams LA, Wree A, Damm G, Seehofer D, Calvente CJ, Povero D, Kisseleva T, Eguchi A, McGeough MD, Hoffman HM, Pelegrin P, Laufs U, Feldstein AE. 2021; Hepatocyte pyroptosis and release of inflammasome particles induce stellate cell activation and liver fibrosis. J Hepatol. 74:156–167. DOI: 10.1016/j.jhep.2020.07.041. PMID: 32763266. PMCID: PMC7749849.
Article46. Jin Y, Huynh DTN, Nguyen TLL, Jeon H, Heo KS. 2020; Therapeutic effects of ginsenosides on breast cancer growth and metastasis. Arch Pharm Res. 43:773–787. DOI: 10.1007/s12272-020-01265-8. PMID: 32839835.
Article47. Zhang Z, Moon R, Thorne JL, Moore JB. 2023; NAFLD and vitamin D: evidence for intersection of microRNA-regulated pathways. Nutr Res Rev. 36:120–139. DOI: 10.1017/S095442242100038X. PMID: 35109946.
Article48. Zhang Q, Yu K, Cao Y, Luo Y, Liu Y, Zhao C. 2021; miR-125b promotes the NF-κB-mediated inflammatory response in NAFLD via directly targeting TNFAIP3. Life Sci. 270:119071. DOI: 10.1016/j.lfs.2021.119071. PMID: 33515562.
Article49. Hu Y, Peng X, Du G, Zhang Z, Zhai Y, Xiong X, Luo X. 2022; MicroRNA-122-5p inhibition improves inflammation and oxidative stress damage in dietary-induced non-alcoholic fatty liver disease through targeting FOXO3. Front Physiol. 13:803445. DOI: 10.3389/fphys.2022.803445. PMID: 35222075. PMCID: PMC8874326. PMID: 8efcb73afd2f4735b6882b87c625e44f.
Article50. Hou X, Yin S, Ren R, Liu S, Yong L, Liu Y, Li Y, Zheng MH, Kunos G, Gao B, Wang H. 2021; Myeloid-cell-specific IL-6 signaling promotes MicroRNA-223-enriched exosome production to attenuate NAFLD-associated fibrosis. Hepatology. 74:116–132. DOI: 10.1002/hep.31658. PMID: 33236445. PMCID: PMC8141545.
Article51. Xu H, Tian Y, Tang D, Zou S, Liu G, Song J, Zhang G, Du X, Huang W, He B, Lin W, Jin L, Huang W, Yang J, Fu X. 2021; An endoplasmic reticulum stress-MicroRNA-26a feedback circuit in NAFLD. Hepatology. 73:1327–1345. DOI: 10.1002/hep.31428. PMID: 32567701.
Article52. Torres LF, Cogliati B, Otton R. 2019; Green tea prevents NAFLD by modulation of miR-34a and miR-194 expression in a high-fat diet mouse model. Oxid Med Cell Longev. 2019:4168380. DOI: 10.1155/2019/4168380. PMID: 31885789. PMCID: PMC6914886.
Article53. Zhao J, Wu Q, Yang T, Nie L, Liu S, Zhou J, Chen J, Jiang Z, Xiao T, Yang J, Chu C. 2022; Gaseous signal molecule SO2 regulates autophagy through PI3K/AKT pathway inhibits cardiomyocyte apoptosis and improves myocardial fibrosis in rats with type II diabetes. Korean J Physiol Pharmacol. 26:541–556. DOI: 10.4196/kjpp.2022.26.6.541. PMID: 36302628. PMCID: PMC9614393.
Article54. Bashiardes S, Shapiro H, Rozin S, Shibolet O, Elinav E. 2016; Non-alcoholic fatty liver and the gut microbiota. Mol Metab. 5:782–794. DOI: 10.1016/j.molmet.2016.06.003. PMID: 27617201. PMCID: PMC5004228. PMID: 829f3bf9256c4ba982f5e506775f3123.
Article55. Zhang X, Coker OO, Chu ES, Fu K, Lau HCH, Wang YX, Chan AWH, Wei H, Yang X, Sung JJY, Yu J. 2021; Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut. 70:761–774. DOI: 10.1136/gutjnl-2019-319664. PMID: 32694178. PMCID: PMC7948195.
Article56. Behary J, Amorim N, Jiang XT, Raposo A, Gong L, McGovern E, Ibrahim R, Chu F, Stephens C, Jebeili H, Fragomeli V, Koay YC, Jackson M, O'Sullivan J, Weltman M, McCaughan G, El-Omar E, Zekry A. 2021; Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat Commun. 12:187. DOI: 10.1038/s41467-020-20422-7. PMID: 33420074. PMCID: PMC7794332. PMID: b2728d3b48ed45bd99530aeb278cdc20.
Article57. Rubio C, Puerto M, García-Rodríquez JJ, Lu VB, García-Martínez I, Alén R, Sanmartín-Salinas P, Toledo-Lobo MV, Saiz J, Ruperez J, Barbas C, Menchén L, Gribble FM, Reimann F, Guijarro LG, Carrascosa JM, Valverde ÁM. 2020; Impact of global PTP1B deficiency on the gut barrier permeability during NASH in mice. Mol Metab. 35:100954. DOI: 10.1016/j.molmet.2020.01.018. PMID: 32244182. PMCID: PMC7082558.
Article58. Wu L, Li J, Feng J, Ji J, Yu Q, Li Y, Zheng Y, Dai W, Wu J, Guo C. 2021; Crosstalk between PPARs and gut microbiota in NAFLD. Biomed Pharmacother. 136:111255. DOI: 10.1016/j.biopha.2021.111255. PMID: 33485064.
Article59. Li DD, Ma JM, Li MJ, Gao LL, Fan YN, Zhang YN, Tao XJ, Yang JJ. 2022; Supplementation of Lycium barbarum polysaccharide combined with aerobic exercise ameliorates high-fat-induced nonalcoholic steatohepatitis via AMPK/PPARα/PGC-1α pathway. Nutrients. 14:3247. DOI: 10.3390/nu14153247. PMID: 35956423. PMCID: PMC9370707. PMID: c18581c6400246a5a032860682c13c9e.
Article60. Yang H, Feng L, Xu L, Jiang D, Zhai F, Tong G, Xing Y. 2022; Intervention of Shugan Xiaozhi decoction on nonalcoholic fatty liver disease via mediating gut-liver axis. Biomed Res Int. 2022:4801695. DOI: 10.1155/2022/4801695. PMID: 35837380. PMCID: PMC9276511.
Article61. Hasan AU, Rahman A, Kobori H. 2019; Interactions between host PPARs and gut microbiota in health and disease. Int J Mol Sci. 20:387. DOI: 10.3390/ijms20020387. PMID: 30658440. PMCID: PMC6359605. PMID: 6d20714c548246c983c10fa37922c936.
Article62. Kanda T, Matsuoka S, Yamazaki M, Shibata T, Nirei K, Takahashi H, Kaneko T, Fujisawa M, Higuchi T, Nakamura H, Matsumoto N, Yamagami H, Ogawa M, Imazu H, Kuroda K, Moriyama M. 2018; Apoptosis and non-alcoholic fatty liver diseases. World J Gastroenterol. 24:2661–2672. DOI: 10.3748/wjg.v24.i25.2661. PMID: 29991872. PMCID: PMC6034146.
Article63. Chang XM, Xiao F, Pan Q, Wang XX, Guo LX. 2021; Sitagliptin attenuates endothelial dysfunction independent of its blood glucose controlling effect. Korean J Physiol Pharmacol. 25:425–437. DOI: 10.4196/kjpp.2021.25.5.425. PMID: 34448460. PMCID: PMC8405439.
Article64. Zhao P, Sun X, Chaggan C, Liao Z, In Wong K, He F, Singh S, Loomba R, Karin M, Witztum JL, Saltiel AR. 2020; An AMPK-caspase-6 axis controls liver damage in nonalcoholic steatohepatitis. Science. 367:652–660. DOI: 10.1126/science.aay0542. PMID: 32029622. PMCID: PMC8012106.
Article65. Jeon H, Jin Y, Myung CS, Heo KS. 2021; Ginsenoside-Rg2 exerts anti-cancer effects through ROS-mediated AMPK activation associated mitochondrial damage and oxidation in MCF-7 cells. Arch Pharm Res. 44:702–712. DOI: 10.1007/s12272-021-01345-3. PMID: 34302638.
Article66. Qiao JT, Cui C, Qing L, Wang LS, He TY, Yan F, Liu FQ, Shen YH, Hou XG, Chen L. 2018; Activation of the STING-IRF3 pathway promotes hepatocyte inflammation, apoptosis and induces metabolic disorders in nonalcoholic fatty liver disease. Metabolism. 81:13–24. DOI: 10.1016/j.metabol.2017.09.010. PMID: 29106945.
Article67. Jin Y, Huynh DTN, Heo KS. 2022; Ginsenoside Rh1 inhibits tumor growth in MDA-MB-231 breast cancer cells via mitochondrial ROS and ER stress-mediated signaling pathway. Arch Pharm Res. 45:174–184. DOI: 10.1007/s12272-022-01377-3. PMID: 35325393.
Article68. Li Y, Xu J, Lu Y, Bian H, Yang L, Wu H, Zhang X, Zhang B, Xiong M, Chang Y, Tang J, Yang F, Zhao L, Li J, Gao X, Xia M, Tan M, Li J. 2021; DRAK2 aggravates nonalcoholic fatty liver disease progression through SRSF6-associated RNA alternative splicing. Cell Metab. 33:2004–2020.e9. DOI: 10.1016/j.cmet.2021.09.008. PMID: 34614409.
Article69. Nasiri-Ansari N, Nikolopoulou C, Papoutsi K, Kyrou I, Mantzoros CS, Kyriakopoulos G, Chatzigeorgiou A, Kalotychou V, Randeva MS, Chatha K, Kontzoglou K, Kaltsas G, Papavassiliou AG, Randeva HS, Kassi E. 2021; Empagliflozin attenuates non-alcoholic fatty liver disease (NAFLD) in high fat diet fed ApoE(-/-) mice by activating autophagy and reducing ER stress and apoptosis. Int J Mol Sci. 22:818. DOI: 10.3390/ijms22020818. PMID: 33467546. PMCID: PMC7829901. PMID: f967148ee656483cac3dae9949ce40ff.
Article70. Pei K, Gui T, Kan D, Feng H, Jin Y, Yang Y, Zhang Q, Du Z, Gai Z, Wu J, Li Y. 2020; An overview of lipid metabolism and nonalcoholic fatty liver disease. Biomed Res Int. 2020:4020249. DOI: 10.1155/2020/4020249. PMID: 32733940. PMCID: PMC7383338.
Article71. Prasomthong J, Limpeanchob N, Daodee S, Chonpathompikunlert P, Tunsophon S. 2022; Hibiscus sabdariffa extract improves hepatic steatosis, partially through IRS-1/Akt and Nrf2 signaling pathways in rats fed a high fat diet. Sci Rep. 12:7022. DOI: 10.1038/s41598-022-11027-9. PMID: 35487948. PMCID: PMC9054782. PMID: 82f7aaf0fccd429a97edf775230404b2.
Article72. Li F, Ye J, Sun Y, Lin Y, Wu T, Shao C, Ma Q, Liao X, Feng S, Zhong B. 2021; Distinct dose-dependent association of free fatty acids with diabetes development in nonalcoholic fatty liver disease patients. Diabetes Metab J. 45:417–429. DOI: 10.4093/dmj.2020.0039. PMID: 33705650. PMCID: PMC8164943. PMID: 8bbe0bc385c949c8aabd39577c08e02b.
Article73. Wang S, Jung S, Ko KS. 2022; Effects of amino acids supplementation on lipid and glucose metabolism in HepG2 cells. Nutrients. 14:3050. DOI: 10.3390/nu14153050. PMID: 35893906. PMCID: PMC9332103. PMID: 9f6073fa2e634f949e14d06940ce2a8f.
Article74. Li R, Li Y, Yang X, Hu Y, Yu H, Li Y. 2022; Reducing VEGFB accelerates NAFLD and insulin resistance in mice via inhibiting AMPK signaling pathway. J Transl Med. 20:341. DOI: 10.1186/s12967-022-03540-2. PMID: 35907871. PMCID: PMC9338666. PMID: 147f4ab94a114d7490bbc87e88dc85d9.
Article75. Xu B, Jiang M, Chu Y, Wang W, Chen D, Li X, Zhang Z, Zhang D, Fan D, Nie Y, Shao F, Wu K, Liang J. 2018; Gasdermin D plays a key role as a pyroptosis executor of non-alcoholic steatohepatitis in humans and mice. J Hepatol. 68:773–782. DOI: 10.1016/j.jhep.2017.11.040. PMID: 29273476.
Article76. Todoric J, Di Caro G, Reibe S, Henstridge DC, Green CR, Vrbanac A, Ceteci F, Conche C, McNulty R, Shalapour S, Taniguchi K, Meikle PJ, Watrous JD, Moranchel R, Najhawan M, Jain M, Liu X, Kisseleva T, Diaz-Meco MT, Moscat J, et al. 2020; Fructose stimulated de novo lipogenesis is promoted by inflammation. Nat Metab. 2:1034–1045. DOI: 10.1038/s42255-020-0261-2. PMID: 32839596. PMCID: PMC8018782.
Article77. Vasques-Monteiro IML, Silva-Veiga FM, Miranda CS, de Andrade Gonçalves ÉCB, Daleprane JB, Souza-Mello V. 2021; A rise in Proteobacteria is an indicator of gut-liver axis-mediated nonalcoholic fatty liver disease in high-fructose-fed adult mice. Nutr Res. 91:26–35. DOI: 10.1016/j.nutres.2021.04.008. PMID: 34130208.
Article78. Daniel PV, Dogra S, Rawat P, Choubey A, Khan AS, Rajak S, Kamthan M, Mondal P. 2021; NF-κB p65 regulates hepatic lipogenesis by promoting nuclear entry of ChREBP in response to a high carbohydrate diet. . J Biol Chem. 296:100714. DOI: 10.1016/j.jbc.2021.100714. PMID: 33930463. PMCID: PMC8144664.
Article79. Sandoval V, Sanz-Lamora H, Marrero PF, Relat J, Haro D. 2021; Lyophilized maqui (Aristotelia chilensis) berry administration suppresses high-fat diet-induced liver lipogenesis through the induction of the nuclear corepressor SMILE. Antioxidants (Basel). 10:637. DOI: 10.3390/antiox10050637. PMID: 33919415. PMCID: PMC8143281. PMID: fa5892aacf3e44c5ab21df0fdf85b37f.
Article80. Luo G, Xiang L, Xiao L. 2022; Acetyl-CoA deficiency is involved in the regulation of iron overload on lipid metabolism in apolipoprotein E knockout mice. Molecules. 27:4966. DOI: 10.3390/molecules27154966. PMID: 35956917. PMCID: PMC9370536. PMID: b088f247d86d468280f0be391f80b844.
Article81. Hardie DG. 2007; AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 8:774–785. DOI: 10.1038/nrm2249. PMID: 17712357.
Article82. Hsu MC, Guo BC, Chen CH, Hu PA, Lee TS. 2021; Apigenin ameliorates hepatic lipid accumulation by activating the autophagy-mitochondria pathway. J Food Drug Anal. 29:240–254. DOI: 10.38212/2224-6614.3269. PMID: 35696209. PMCID: PMC9261827.
Article83. Shih PH, Shiue SJ, Chen CN, Cheng SW, Lin HY, Wu LW, Wu MS. 2021; Fucoidan and fucoxanthin attenuate hepatic steatosis and inflammation of NAFLD through modulation of leptin/adiponectin axis. Mar Drugs. 19:148. DOI: 10.3390/md19030148. PMID: 33809062. PMCID: PMC8001566. PMID: 53f4c3f331c041e385653fb91ea1a0e9.
Article84. Barbier-Torres L, Fortner KA, Iruzubieta P, Delgado TC, Giddings E, Chen Y, Champagne D, Fernández-Ramos D, Mestre D, Gomez-Santos B, Varela-Rey M, de Juan VG, Fernández-Tussy P, Zubiete-Franco I, García-Monzón C, González-Rodríguez Á, Oza D, Valença-Pereira F, Fang Q, Crespo J, et al. 2020; Silencing hepatic MCJ attenuates non-alcoholic fatty liver disease (NAFLD) by increasing mitochondrial fatty acid oxidation. Nat Commun. 11:3360. DOI: 10.1038/s41467-020-16991-2. PMID: 32620763. PMCID: PMC7334216. PMID: f4585c15ec8641c2ad4a105f86c1a9ca.
Article85. Lu Q, Tian X, Wu H, Huang J, Li M, Mei Z, Zhou L, Xie H, Zheng S. 2021; Metabolic changes of hepatocytes in NAFLD. Front Physiol. 12:710420. DOI: 10.3389/fphys.2021.710420. PMID: 34526911. PMCID: PMC8437340. PMID: 34d4a99eb3544a13a215b9f7ae621c2d.
Article86. Li H, Xu Q, Xu C, Hu Y, Yu X, Zhao K, Li M, Li M, Xu J, Kuang H. 2021; Bicyclol regulates hepatic gluconeogenesis in rats with type 2 diabetes and non-alcoholic fatty liver disease by inhibiting inflammation. Front Pharmacol. 12:644129. DOI: 10.3389/fphar.2021.644129. PMID: 34093184. PMCID: PMC8175979. PMID: 72ec72ee182a4c92b90af230041a565d.
Article87. Im SS, Kim MY, Kwon SK, Kim TH, Bae JS, Kim H, Kim KS, Oh GT, Ahn YH. 2011; Peroxisome proliferator-activated receptor {alpha} is responsible for the up-regulation of hepatic glucose-6-phosphatase gene expression in fasting and db/db mice. J Biol Chem. 286:1157–1164. DOI: 10.1074/jbc.M110.157875. PMID: 21081500. PMCID: PMC3020722.
Article88. Song D, Yin L, Wang C, Wen X. 2020; Zhenqing recipe attenuates non-alcoholic fatty liver disease by regulating the SIK1/CRTC2 signaling in experimental diabetic rats. BMC Complement Med Ther. 20:27. DOI: 10.1186/s12906-019-2811-2. PMID: 32020874. PMCID: PMC7076741. PMID: 1471a750307546c5b6034c75bc2bf4fb.
Article89. Choudhury M, Qadri I, Rahman SM, Schroeder-Gloeckler J, Janssen RC, Friedman JE. 2011; C/EBPβ is AMP kinase sensitive and up-regulates PEPCK in response to ER stress in hepatoma cells. Mol Cell Endocrinol. 331:102–108. DOI: 10.1016/j.mce.2010.08.014. PMID: 20797423. PMCID: PMC2981635.
Article90. Phung HH, Lee CH. 2022; Mouse models of nonalcoholic steatohepatitis and their application to new drug development. Arch Pharm Res. 45:761–794. DOI: 10.1007/s12272-022-01410-5. PMID: 36318445.
Article91. Khan MS, Lee C, Kim SG. 2022; Non-alcoholic fatty liver disease and liver secretome. Arch Pharm Res. 45:938–963. DOI: 10.1007/s12272-022-01419-w. PMID: 36441472. PMCID: PMC9703441.
Article92. Shaaban HH, Alzaim I, El-Mallah A, Aly RG, El-Yazbi AF, Wahid A. 2022; Metformin, pioglitazone, dapagliflozin and their combinations ameliorate manifestations associated with NAFLD in rats via anti-inflammatory, anti-fibrotic, anti-oxidant and anti-apoptotic mechanisms. Life Sci. 308:120956. DOI: 10.1016/j.lfs.2022.120956. PMID: 36103959.
Article93. Liu B, Xiang L, Ji J, Liu W, Chen Y, Xia M, Liu Y, Liu W, Zhu P, Jin Y, Han Y, Lu J, Li X, Zheng M, Lu Y. 2021; Sparcl1 promotes nonalcoholic steatohepatitis progression in mice through upregulation of CCL2. J Clin Invest. 131:e144801. DOI: 10.1172/JCI144801. PMID: 34651580. PMCID: PMC8516465.
Article94. Huang S, Wu Y, Zhao Z, Wu B, Sun K, Wang H, Qin L, Bai F, Leng Y, Tang W. 2021; A new mechanism of obeticholic acid on NASH treatment by inhibiting NLRP3 inflammasome activation in macrophage. Metabolism. 120:154797. DOI: 10.1016/j.metabol.2021.154797. PMID: 33984334.95. Puengel T, Lefere S, Hundertmark J, Kohlhepp M, Penners C, Van de Velde F, Lapauw B, Hoorens A, Devisscher L, Geerts A, Boehm S, Zhao Q, Krupinski J, Charles ED, Zinker B, Tacke F. 2022; Combined therapy with a CCR2/CCR5 antagonist and FGF21 analogue synergizes in ameliorating steatohepatitis and fibrosis. Int J Mol Sci. 23:6696. DOI: 10.3390/ijms23126696. PMID: 35743140. PMCID: PMC9224277. PMID: 8267c70907c64c32a767d42485b7e4e4.
Article96. Crengle S. 2020; Comment on: Jaine et al Cost-effectiveness of a low-dose computed tomography screening programme for lung cancer in New Zealand. Lung Cancer 2018, 124, 233-240. 2018/10/01. DOI: 10.1016/j.lungcan.2018.08.004. Lung Cancer. 145:219–220. DOI: 10.1016/j.lungcan.2018.08.004. PMID: 30268467.
Article97. Ala M, Eftekhar SP. 2023; Weight loss breaks the bond between nonalcoholic fatty liver disease and cardiovascular diseases: a clinical and epidemiological perspective. Obes Rev. 24:e13563. DOI: 10.1111/obr.13563. PMID: 36951144.
Article98. Ma Z, Jin K, Yue M, Chen X, Chen J. 2023; Research progress on the GIP/GLP-1 receptor coagonist tirzepatide, a rising star in type 2 diabetes. J Diabetes Res. 2023:5891532. DOI: 10.1155/2023/5891532. PMID: 37096236. PMCID: PMC10122586. PMID: ebf346f2c12c4f8c8785124c0ae0cc3d.
Article99. Valenzuela-Vallejo L, Guatibonza-García V, Mantzoros CS. 2022; Recent guidelines for non-alcoholic fatty liver disease (NAFLD)/fatty liver disease (FLD): are they already outdated and in need of supplementation? Metabolism. 136:155248. DOI: 10.1016/j.metabol.2022.155248. PMID: 35803320.100. Giugliano D, Scappaticcio L, Longo M, Caruso P, Maiorino MI, Bellastella G, Ceriello A, Chiodini P, Esposito K. 2021; GLP-1 receptor agonists and cardiorenal outcomes in type 2 diabetes: an updated meta-analysis of eight CVOTs. Cardiovasc Diabetol. 20:189. DOI: 10.1186/s12933-021-01366-8. PMID: 34526024. PMCID: PMC8442438. PMID: 350ec22390e74c53b7c53d0cd7a32144.
Article101. Greenan J. 1984; Cardiac dysrhythmias and heart rate changes at induction of anaesthesia: a comparison of two intravenous anticholinergics. Acta Anaesthesiol Scand. 28:182–184. DOI: 10.1111/j.1399-6576.1984.tb02037.x. PMID: 6375235.
Article102. Tacke F, Puengel T, Loomba R, Friedman SL. 2023; An integrated view of anti-inflammatory and antifibrotic targets for the treatment of NASH. J Hepatol. S0168-8278(23)00218-0. DOI: 10.1016/j.jhep.2023.03.038. PMID: 37061196.
Article103. Pennisi G, Celsa C, Enea M, Vaccaro M, Di Marco V, Ciccioli C, Infantino G, La Mantia C, Parisi S, Vernuccio F, Craxì A, Cammà C, Petta S. 2022; Effect of pharmacological interventions and placebo on liver histology in nonalcoholic steatohepatitis: a network meta-analysis. Nutr Metab Cardiovasc Dis. 32:2279–2288. DOI: 10.1016/j.numecd.2022.07.001. PMID: 35970684.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Should you advocate for hepatocellular carcinomasurveillance in patients with alcohol-related liverdisease or non-alcoholic fatty liver disease?
- KASL Clinical Practice Guidelines: Management of Alcoholic Liver Disease
- Histologic Risk Factor for Mortality and Development of Severe Liver Disease in Biopsy-proven Non-alcoholic Fatty Liver Disease
- The Risk of Abdominal Obesity according to the Degree of Non-Alcoholic Fatty Liver Disease in Korean Men
- How to optimize the outcome of liver transplantation for non-alcoholic fatty liver disease