Ann Lab Med.
2018 May;38(3):249-254. 10.3343/alm.2018.38.3.249.
Budget Impact of the Accreditation Program for Clinical Laboratories on Colorectal Cancer Screening via Fecal Immunochemical Testing: Results from the National Cancer Screening Program in Korea
- Affiliations
-
- 1National Cancer Control Institute, National Cancer Center, Goyang, Korea.
- 2Department of Laboratory Medicine and Genetics, Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Korea. cecilia@schmc.ac.kr
- 3Department of Laboratory Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 4Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 5Department of Laboratory Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2403459
- DOI: http://doi.org/10.3343/alm.2018.38.3.249
Abstract
- BACKGROUND
False-positive fecal immunochemical test (FIT) results in the National Cancer Screening Program (NCSP) for colorectal cancer may lead to unnecessary procedures, such as colonoscopies, increasing the medical costs. We estimated reductions in the cost of National Health Insurance according to the accreditation status of screening facilities participating in the NCSP for colorectal cancer.
METHODS
We used data collected between 2007 and 2010 from NCSP and the Korea Central Cancer Registry to identify patients with colorectal cancer. We also ascertained the history of the accreditation of each facility by the Korean Laboratory Accreditation Program (KLAP). Budget impact was defined as a reduction in medical costs achieved when the false-positive rate of the non-accredited facilities decreased relative to that of the accredited facilities.
RESULTS
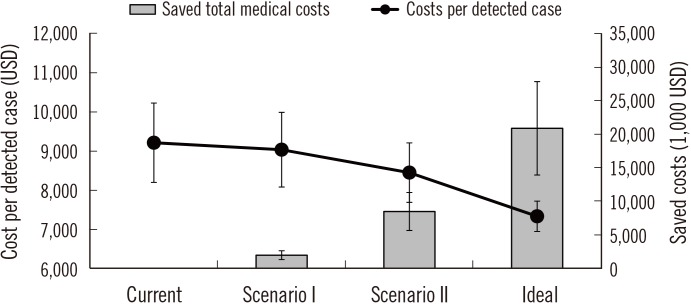
A total of 3,285 screening facilities participated in the NCSP. Of these, 196 were accredited by the KLAP. The false-positive rate of the accredited facilities was 2.47%, and that of the non-accredited facilities was 6.83%. Medical costs were estimated to be reduced by approximately 19 million US dollars (USD), and the cost of detecting one case of colorectal cancer was estimated to decrease from 9,212 USD to 7,332 USD if the false-positive rate of non-accredited facilities were decreased to that of the accredited facilities. Clinics were estimated to have the largest associated cost reduction.
CONCLUSIONS
Quality assurance in clinical laboratories could lower false-positive rates and prevent the use of unnecessary procedures, ensuring patient safety and increasing the cost-effectiveness of FIT screening in the NCSP for colorectal cancer.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Korean Clinical Laboratory Accreditation Program Quality Standards for Laboratory Management: Identifying a Compliance Gap with World Health Organization Quality System Essentials
Byung Ryul Jeon, Chiho Yoon, Mi-Ae Jang, Sung Ran Cho, Sollip Kim, You Kyoung Lee
Lab Med Online. 2020;10(2):152-159. doi: 10.3343/lmo.2020.10.2.152.
Reference
-
1. Jung KW, Won YJ, Oh CM, Kong HJ, Lee DH, Lee KH. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2014. Cancer Res Treat. 2017; 49:292–305. PMID: 28279062.2. Park MJ, Choi KS, Lee YK, Jun JK, Lee HY. A comparison of qualitative and quantitative fecal immunochemical tests in the Korean national colorectal cancer screening program. Scand J Gastroenterol. 2012; 47:461–466. PMID: 22428929.3. Young GP, Symonds EL, Allison JE, Cole SR, Fraser CG, Halloran SP, et al. Advances in fecal occult blood tests: the FIT revolution. Dig Dis Sci. 2015; 60:609–622. PMID: 25492500.4. Suh M, Song S, Cho HN, Park B, Jun JK, Choi E, et al. Trends in participation rates for the National Cancer Screening Program in Korea, 2002-2012. Cancer Res Treat. 2017; 49:798–806. PMID: 27857022.5. Shin BM, Chae SL, Min WK, Lee WG, Lim YA, Lee DH, et al. The implementation and effects of a clinical laboratory accreditation program in Korea from 1999 to 2006. Korean J Lab Med. 2009; 29:163–170. PMID: 19411785.6. Yoo SJ, Cha YJ, Min WK, Lee YK, Chae SL, Shin BM, et al. Current status of external quality assessment of fecal occult blood test. Korean J Lab Med. 2010; 30:726–733. PMID: 21157162.7. Jeon CH, Lee AJ, Kim KD. Annual report on external quality assessment scheme for urinalysis and faecal occult blood testing in Korea (2014). J Lab Med Qual Assur. 2015; 37:179–189.8. Cha JM, Moon JS, Chung IK, Kim JO, Im JP, Cho YK, et al. National Endoscopy Quality Improvement Program remains suboptimal in Korea. Gut Liver. 2016; 10:699–705. PMID: 27282270.9. Lin JS, Piper MA, Perdue LA, Rutter CM, Webber EM, O'Connor E, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Service Task Force. JAMA. 2016; 315:2576–2594. PMID: 27305422.10. Jang MA, Yoon YA, Song J, Kim JH, Min WK, Lee JS, et al. Effect of accreditation on accuracy of diagnostic tests in medical laboratories. Ann Lab Med. 2017; 37:213–222. PMID: 28224767.11. Shin A, Choi KS, Jun JK, Noh DK, Suh M, Jung KW, et al. Validity of fecal occult blood test in the National Cancer Screening Program, Korea. PLoS One. 2013; 8:e79292. PMID: 24260189.12. Lee HY, Park EC, Jun JK, Choi KS, Hahm MI. Comparing upper gastrointestinal X-ray and endoscopy for gastric cancer diagnosis in Korea. World J Gastroenterol. 2010; 16:245–250. PMID: 20066745.13. Choi KS, Lee HY, Jun JK, Shin A, Park EC. Adherence to follow-up after a positive fecal occult blood test in an organized colorectal cancer screening program in Korea, 2004-2008. J Gastroenterol Hepatol. 2012; 27:1070–1077. PMID: 22004224.14. International Society for Quality in Health Care and World Health Organization. Department of Organization of Health Services Delivery. Quality and accreditation in health care services: a global review. Geneva: World Health Organization;2003.15. Robertson DJ, Kaminski MF, Bretthauer M. Effectiveness, training and quality assurance of colonoscopy screening for colorectal cancer. Gut. 2015; 64:982–990. PMID: 25804631.16. Suh M, Choi KS, Park B, Lee YY, Jun JK, Lee DH, et al. Trends in cancer screening rates among Korean men and women: results of the Korean National Cancer Screening Survey, 2004-2013. Cancer Res Treat. 2016; 48:1–10. PMID: 25943324.17. Knudsen AB, Zauber AG, Rutter CM, Naber SK, Doria-Rose VP, Pabiniak C, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA. 2016; 315:2595–2609. PMID: 27305518.18. Lee KS. Cost-effectiveness of colorectal cancer screening interventions with their effects on health disparity being considered. Cancer Res Treat. 2016; 48:1010–1019. PMID: 26727714.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Population Screening for Colorectal Cancer Means Getting FIT: The Past, Present, and Future of Colorectal Cancer Screening Using the Fecal Immunochemical Test for Hemoglobin (FIT)
- Current Status of Colorectal Cancer and Its Public Health Burden in Thailand
- National cancer screening program for colorectal cancer in Korea
- Increase in the Colorectal Cancer Screening Rate by a Round-Mailed Fecal Immunochemical Testing Kit and Associated Factors in Underserved Regions of Korea: A Community-Based Intervention Study
- Evidence-based National Cancer Screening program of Korea