Cancer Res Treat.
2015 Oct;47(4):630-637. 10.4143/crt.2014.244.
Pemetrexed Singlet Versus Nonpemetrexed-Based Platinum Doublet as Second-Line Chemotherapy after First-Line Epidermal Growth Factor Receptor (EGFR) Tyrosine Kinase Inhibitor Failure in Non-small Cell Lung Cancer Patients with EGFR Mutations
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea. bhumsuk@snu.ac.kr
- 2Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 3Department of Internal Medicine, Seoul National University Boramae Medical Center, Seoul, Korea.
- KMID: 2403380
- DOI: http://doi.org/10.4143/crt.2014.244
Abstract
- PURPOSE
Platinum-based doublet chemotherapy is the treatment of choice for patients with non-small cell lung cancer (NSCLC); however, the role of a platinum-based doublet as second-line therapy after failure of an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) for NSCLC patients has not yet been elucidated. The purpose of this study was to compare the clinical efficacy of pemetrexed versus a platinum-based doublet as second-line therapy after failure of EGFR TKI used as first-line therapy for NSCLC patients with EGFR mutations.
MATERIALS AND METHODS
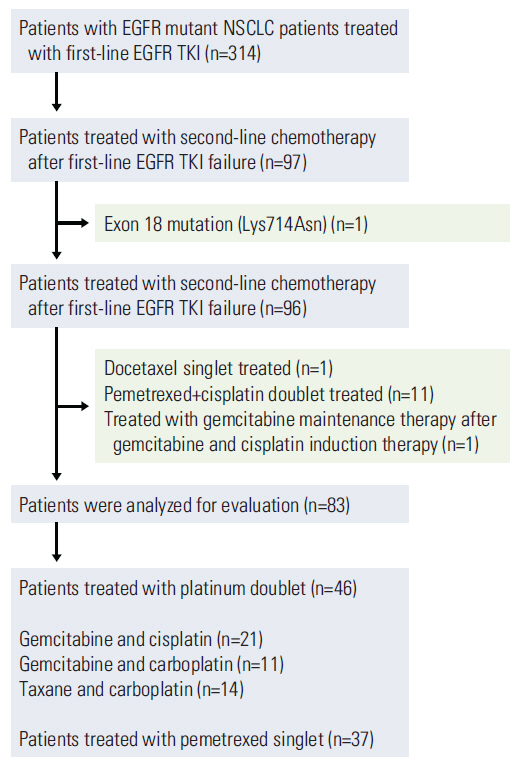
We designed a multicenter retrospective cohort study of 314 NSCLC patients with EGFR mutations who received an EGFR TKI as first-line palliative chemotherapy. Our analysis included 83 patients who failed EGFR TKI therapy and received second-line cytotoxic chemotherapy.
RESULTS
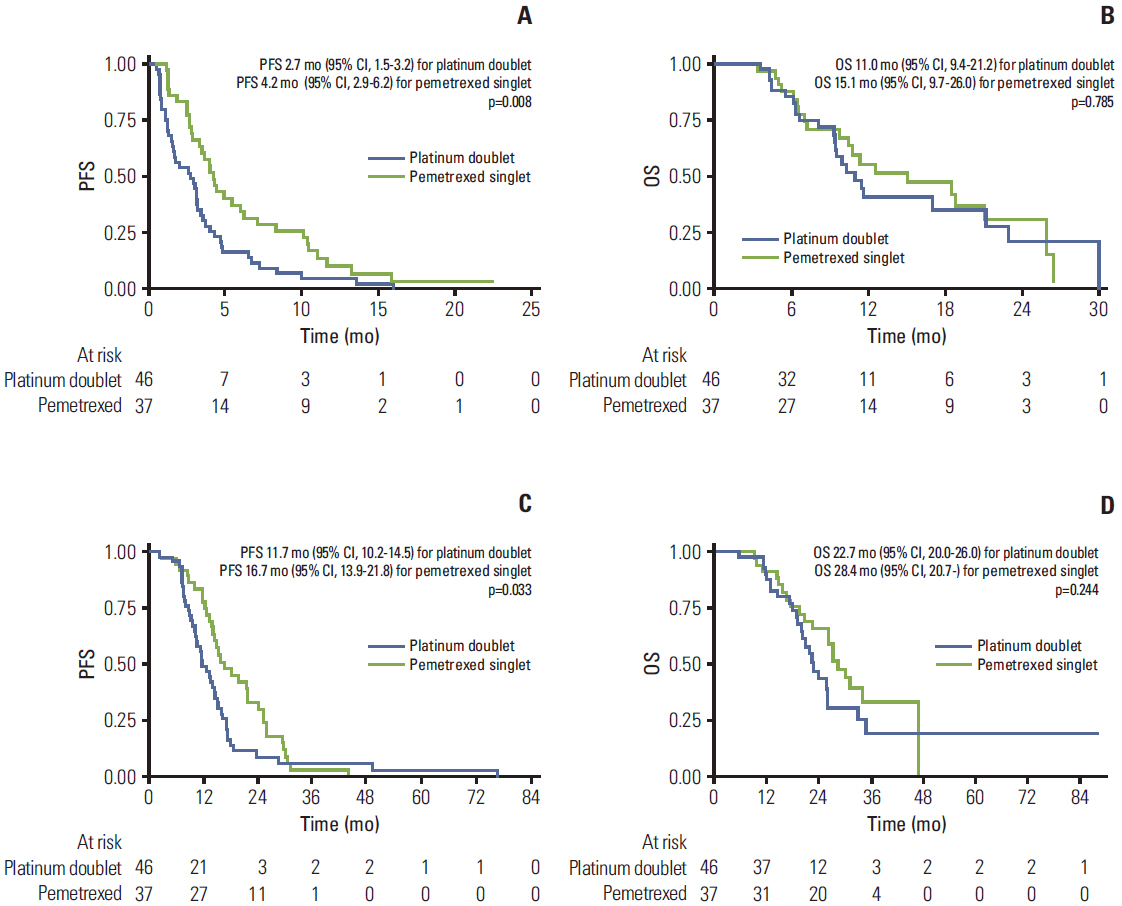
Forty-six patients were treated using a platinum-based doublet and 37 patients were treated using singlet pemetrexed. The overall response rates of patients receiving a platinum-based doublet and patients receiving pemetrexed were17.4% and 32.4%, respectively (p=0.111). The median progression-free survival (PFS) of patients receiving pemetrexed was significantly longer than that of patients receiving a platinum-based doublet (4.2 months vs. 2.7 months, respectively; p=0.008). The hazard ratio was 0.54 (95% confidence interval, 0.34 to 0.86; p=0.009).
CONCLUSION
Our retrospective analysis found that second-line pemetrexed singlet therapy provided significantly prolonged PFS compared to second-line platinum-based doublet chemotherapy for NSCLC patients with EGFR mutations who failed first-line EGFR TKI. Conduct of prospective studies for confirmation of our results is warranted.
MeSH Terms
-
Carcinoma, Non-Small-Cell Lung*
Cohort Studies
Disease-Free Survival
Drug Therapy*
Epidermal Growth Factor*
Humans
Phosphotransferases*
Platinum*
Prospective Studies
Protein-Tyrosine Kinases
Receptor, Epidermal Growth Factor*
Retrospective Studies
Epidermal Growth Factor
Phosphotransferases
Platinum
Protein-Tyrosine Kinases
Receptor, Epidermal Growth Factor
Figure
Reference
-
References
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012; 62:10–29.
Article2. Novello S, Le Chevalier T. Chemotherapy for non-small-cell lung cancer. Part 1: early-stage disease. Oncology (Williston Park). 2003; 17:357–64.3. Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002; 346:92–8.
Article4. Azzoli CG, Baker S Jr, Temin S, Pao W, Aliff T, Brahmer J, et al. American Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-smallcell lung cancer. J Clin Oncol. 2009; 27:6251–66.
Article5. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010; 362:2380–8.
Article6. Han JY, Park K, Kim SW, Lee DH, Kim HY, Kim HT, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012; 30:1122–8.
Article7. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010; 11:121–8.
Article8. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012; 13:239–46.9. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011; 12:735–42.
Article10. Hirsch FR, Bunn PA Jr. EGFR testing in lung cancer is ready for prime time. Lancet Oncol. 2009; 10:432–3.
Article11. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004; 350:2129–39.
Article12. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: non-small cell lung cancer. ver. 4.2014. Fort Washington, PA: National Comprehensive Cancer Network;2014.13. Keam B, Kim DW, Park JH, Lee JO, Kim TM, Lee SH, et al. Rare and complex mutations of epidermal growth factor receptor, and efficacy of tyrosine kinase inhibitor in patients with non-small cell lung cancer. Int J Clin Oncol. 2014; 19:594–600.
Article14. Kim YT, Kim TY, Lee DS, Park SJ, Park JY, Seo SJ, et al. Molecular changes of epidermal growth factor receptor (EGFR) and KRAS and their impact on the clinical outcomes in surgically resected adenocarcinoma of the lung. Lung Cancer. 2008; 59:111–8.
Article15. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.
Article16. Gridelli C, Perrone F, Gallo C, Cigolari S, Rossi A, Piantedosi F, et al. Chemotherapy for elderly patients with advanced non-small-cell lung cancer: the Multicenter Italian Lung Cancer in the Elderly Study (MILES) phase III randomized trial. J Natl Cancer Inst. 2003; 95:362–72.
Article17. Lilenbaum R, Zukin M, Pereira JR, Barrios CH, De Albuquerque Ribeiro R, de Mendonça Beato CA, et al. A randomized phase III trial of single-agent pemetrexed (P) versus carboplatin and pemetrexed (CP) in patients with advanced non-small cell lung cancer (NSCLC) and performance status (PS) of 2. J Clin Oncol. 2012; 30 Suppl:7506.
Article18. Roth BJ, Krilov L, Adams S, Aghajanian CA, Bach P, Braiteh F, et al. Clinical cancer advances 2012: annual report on progress against cancer from the american society of clinical oncology. J Clin Oncol. 2013; 31:131–61.
Article19. Zukin M, Barrios CH, Pereira JR, Ribeiro Rde A, Beato CA, do Nascimento YN, et al. Randomized phase III trial of singleagent pemetrexed versus carboplatin and pemetrexed in patients with advanced non-small-cell lung cancer and Eastern Cooperative Oncology Group performance status of 2. J Clin Oncol. 2013; 31:2849–53.
Article20. Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapynaive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008; 26:3543–51.
Article21. Douillard JY, Shepherd FA, Hirsh V, Mok T, Socinski MA, Gervais R, et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: data from the randomized phase III INTEREST trial. J Clin Oncol. 2010; 28:744–52.
Article22. Park JH, Lee SH, Keam B, Kim TM, Kim DW, Yang SC, et al. EGFR mutations as a predictive marker of cytotoxic chemotherapy. Lung Cancer. 2012; 77:433–7.
Article23. Huang CL, Yokomise H, Kobayashi S, Fukushima M, Hitomi S, Wada H. Intratumoral expression of thymidylate synthase and dihydropyrimidine dehydrogenase in non-small cell lung cancer patients treated with 5-FU-based chemotherapy. Int J Oncol. 2000; 17:47–54.
Article24. Nakagawa T, Tanaka F, Otake Y, Yanagihara K, Miyahara R, Matsuoka K, et al. Prognostic value of thymidylate synthase expression in patients with p-stage I adenocarcinoma of the lung. Lung Cancer. 2002; 35:165–70.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pemetrexed Continuation Maintenance versus Conventional Platinum-Based Doublet Chemotherapy in EGFR-Negative Lung Adenocarcinoma: Retrospective Analysis
- Does the efficacy of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor differ according to the type of EGFR mutation in non-small cell lung cancer?
- Treatment of Non-small Cell Lung Carcinoma after Failure of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor
- A Case of Patient with Lung Adenocarcinoma with Double Rare EGFR Mutation of G719C and L861Q
- Anti-Cancer Therapy of Advanced Lung Cancer in Elderly Patients