Healthc Inform Res.
2017 Jul;23(3):199-207. 10.4258/hir.2017.23.3.199.
Pilot Algorithm Designed to Help Early Detection of HMG-CoA Reductase Inhibitor-Induced Hepatotoxicity
- Affiliations
-
- 1Division of Biomedical Informatics, Systems Biomedical Informatics Research Centre, Seoul National University College of Medicine, Seoul, Korea.
- 2Cipherome Inc., Seoul, Korea.
- 3Department of Medical Informatics, College of Medicine, The Catholic University of Korea, Seoul, Korea. iychoi@catholic.ac.kr
- 4Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2403310
- DOI: http://doi.org/10.4258/hir.2017.23.3.199
Abstract
OBJECTIVES
To enable early detection of adverse drug reactions (ADRs) in patients using HMG-CoA reductase inhibitors (statins), we developed an algorithm that automatically detects liver injury caused by statins from Electronic Medical Record (EMR) data. We verified the performance of our algorithm through manual ADR assessment and a direct chart review.
METHODS
The subjects in this study were patients who had been prescribed a statin for the first time among outpatients in Seoul St. Mary's Hospital in Korea between January 2009 and December 2012. We extracted basic information about the patients, including laboratory information, underlying disease, diagnosis information, prescription information, and concomitant drugs. We developed an automatic ADR detection algorithm by using EMR data. We validated the results of the algorithm through a chart review.
RESULTS
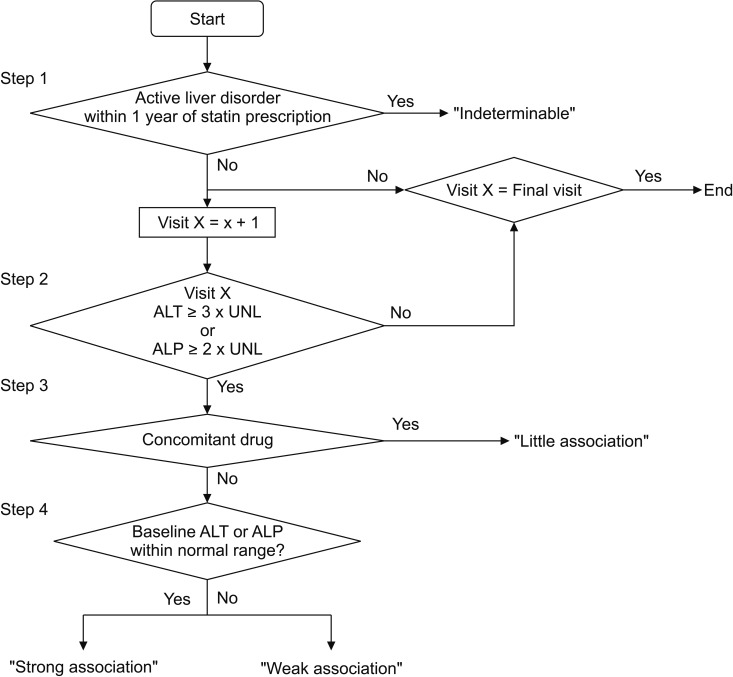
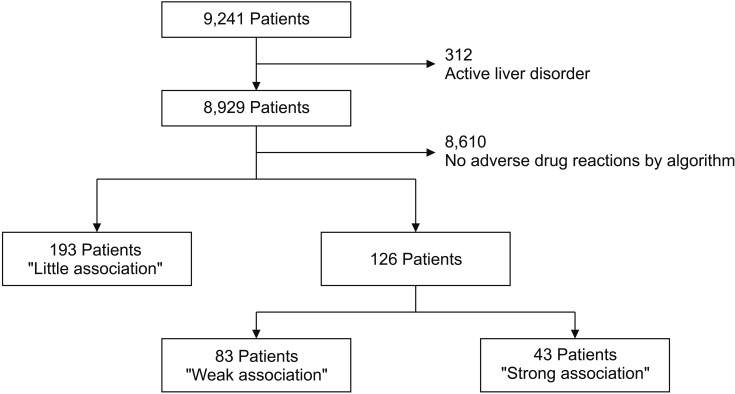
We developed the algorithm to assess ADR occurrences based on alanine transaminase (ALT) and alkaline phosphatase (ALP) levels. According to the proposed algorithm, any of these result options could be attained: ADR-free, little association, strong association, and weak association or indeterminable. The results of the ADR assessments obtained using the proposed algorithm showed that the data of 126 patients (1.4% of all 9,241 patients) included suspicious figures, thus indicating the possibility of an ADR. In the EMR chart review for verifying the algorithm, ADRs of 33 patients were not associated with statin use; therefore, the ADR occurrence rate was found to be 1.0% (93/9,241). Therefore, the positive predictive value was calculated to be 73.8% (93/126; 95% confidence interval, 69.2%-77.6%). No differences were observed between statin types (p = 0.472).
CONCLUSIONS
For early detection of statin-induced liver injury, we developed an automatic ADR assessment algorithm. We expect that algorithms that are more reliable can be developed if we conduct supplement clinical studies with a focus on adverse drug effects.
Keyword
MeSH Terms
-
Alanine Transaminase
Alkaline Phosphatase
Diagnosis
Drug-Induced Liver Injury
Drug-Related Side Effects and Adverse Reactions
Electronic Health Records
Humans
Hydroxymethylglutaryl-CoA Reductase Inhibitors
Korea
Liver
Outpatients
Oxidoreductases*
Prescriptions
Seoul
Alanine Transaminase
Alkaline Phosphatase
Hydroxymethylglutaryl-CoA Reductase Inhibitors
Oxidoreductases
Figure
Reference
-
1. Shepherd G, Mohorn P, Yacoub K, May DW. Adverse drug reaction deaths reported in United States vital statistics, 1999-2006. Ann Pharmacother. 2012; 46(2):169–175. PMID: 22253191.
Article2. Brown JS, Kulldorff M, Chan KA, Davis RL, Graham D, Pettus PT, et al. Early detection of adverse drug events within population-based health networks: application of sequential testing methods. Pharmacoepidemiol Drug Saf. 2007; 16(12):1275–1284. PMID: 17955500.
Article3. Park IW, Sheen SS, Yoon D, Lee SH, Shin GT, Kim H, et al. Onset time of hyperkalaemia after angiotensin receptor blocker initiation: when should we start serum potassium monitoring? J Clin Pharm Ther. 2014; 39(1):61–68. PMID: 24262001.
Article4. Jha AK, Kuperman GJ, Teich JM, Leape L, Shea B, Rittenberg E, et al. Identifying adverse drug events: development of a computer-based monitor and comparison with chart review and stimulated voluntary report. J Am Med Inform Assoc. 1998; 5(3):305–314. PMID: 9609500.
Article5. Haerian K, Varn D, Vaidya S, Ena L, Chase HS, Friedman C. Detection of pharmacovigilance-related adverse events using electronic health records and automated methods. Clin Pharmacol Ther. 2012; 92(2):228–234. PMID: 22713699.
Article6. Hannan TJ. Detecting adverse drug reactions to improve patient outcomes. Int J Med Inform. 1999; 55(1):61–64. PMID: 10471241.
Article7. Grundy SM. HMG-CoA reductase inhibitors for treatment of hypercholesterolemia. N Engl J Med. 1988; 319(1):24–33. PMID: 3288867.
Article8. Kim HS, Kim H, Lee H, Park B, Park S, Lee SH, et al. Analysis and comparison of statin prescription patterns and outcomes according to clinical department. J Clin Pharm Ther. 2016; 41(1):70–77. PMID: 26791968.
Article9. Kim HS, Lee H, Park B, Park S, Kim H, Lee SH, et al. Comparative analysis of the efficacy of low- and moderate-intensity statins in Korea. Int J Clin Pharmacol Ther. 2016; 54(11):864–871. PMID: 27487366.10. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014; 129(25 Suppl 2):S1–S45. PMID: 24222016.11. Kim HS, Lee SH, Kim H, Lee SH, Cho JH, Lee H, et al. Statin-related aminotransferase elevation according to baseline aminotransferases level in real practice in Korea. J Clin Pharm Ther. 2016; 41(3):266–272. PMID: 27015878.
Article12. Benichou C. Criteria of drug-induced liver disorders: report of an international consensus meeting. J Hepatol. 1990; 11(2):272–276. PMID: 2254635.13. Bellosta S, Paoletti R, Corsini A. Safety of statins: focus on clinical pharmacokinetics and drug interactions. Circulation. 2004; 109(23 Suppl 1):III50–III57. PMID: 15198967.14. Tantikul C, Dhana N, Jongjarearnprasert K, Visitsunthorn N, Vichyanond P, Jirapongsananuruk O. The utility of the World Health Organization-The Uppsala Monitoring Centre (WHO-UMC) system for the assessment of adverse drug reactions in hospitalized children. Asian Pac J Allergy Immunol. 2008; 26(2-3):77–82. PMID: 19054924.15. Knopp RH. Drug treatment of lipid disorders. N Engl J Med. 1999; 341(7):498–511. PMID: 10441607.
Article16. Bhardwaj SS, Chalasani N. Lipid-lowering agents that cause drug-induced hepatotoxicity. Clin Liver Dis. 2007; 11(3):597–613. PMID: 17723922.
Article17. Russo MW, Hoofnagle JH, Gu J, Fontana RJ, Barnhart H, Kleiner DE, Chalasani N, Bonkovsky HL. Spectrum of statin hepatotoxicity: experience of the drug-induced liver injury network. Hepatology. 2014; 60(2):679–686. PMID: 24700436.
Article18. Farmer JA, Torre-Amione G. Comparative tolerability of the HMG-CoA reductase inhibitors. Drug Saf. 2000; 23(3):197–213. PMID: 11005703.
Article19. Tolman KG. Defining patient risks from expanded preventive therapies. Am J Cardiol. 2000; 85(12A):15E–19E.
Article20. Sniderman AD. Is there value in liver function test and creatine phosphokinase monitoring with statin use? Am J Cardiol. 2004; 94(9A):30F–34F. PMID: 15219504.
Article21. Reuben A, Koch DG, Lee WM. Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010; 52(6):2065–2076. PMID: 20949552.
Article22. Russo MW, Scobey M, Bonkovsky HL. Drug-induced liver injury associated with statins. Semin Liver Dis. 2009; 29(4):412–422. PMID: 19826975.
Article23. Tavassoli N, Duchayne E, Sadaba B, Desboeuf K, Sommet A, Lapeyre-Mestre M, et al. Detection and incidence of drug-induced agranulocytosis in hospital: a prospective analysis from laboratory signals. Eur J Clin Pharmacol. 2007; 63(3):221–228. PMID: 17225990.
Article24. Bagheri H, Michel F, Lapeyre-Mestre M, Lagier E, Cambus JP, Valdiguie P, et al. Detection and incidence of drug-induced liver injuries in hospital: a prospective analysis from laboratory signals. Br J Clin Pharmacol. 2000; 50(5):479–484. PMID: 11069443.
Article25. Robb MA, Racoosin JA, Sherman RE, Gross TP, Ball R, Reichman ME, et al. The US Food and Drug Administration's Sentinel Initiative: expanding the horizons of medical product safety. Pharmacoepidemiol Drug Saf. 2012; 21(Suppl 1):9–11. PMID: 22262587.
Article26. Yoon D, Park MY, Choi NK, Park BJ, Kim JH, Park RW. Detection of adverse drug reaction signals using an electronic health records database: Comparison of the Laboratory Extreme Abnormality Ratio (CLEAR) algorithm. Clin Pharmacol Ther. 2012; 91(3):467–474. PMID: 22237257.
Article27. Klintmalm GB, Iwatsuki S, Starzl TE. Cyclosporin A hepatotoxicity in 66 renal allograft recipients. Transplantation. 1981; 32(6):488–489. PMID: 7041349.
Article28. Wagoner LE, Olsen SL, Bristow MR, O'Connell JB, Taylor DO, Lappe DL, et al. Cyclophosphamide as an alternative to azathioprine in cardiac transplant recipients with suspected azathioprine-induced hepatotoxicity. Transplantation. 1993; 56(6):1415–1418. PMID: 8279012.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Myotonic Potentials in the Myopathy Induced by HMG-CoA Reductase Inhibitor
- A Case of Acquired Ichthyosis Developed During Cholesterol-lowering Treatment
- The Effects of Long Term Use of HMG-CoA Reductase Inhibitor on the Level of Lp (a)
- HMG CoA Reductase Inhibitors Inhibit HCV RNA Replication of HCV Genotype 1b but Not 2a
- Ichthysiform Skin Eruptions Possibly Due to Lovastatin(Mevacor)