J Pathol Transl Med.
2018 Jan;52(1):9-13. 10.4132/jptm.2017.07.26.
Programmed Death-Ligand 1 Expression and Its Correlation with Lymph Node Metastasis in Papillary Thyroid Carcinoma
- Affiliations
-
- 1Department of Pathology, Gyeongsang National University Changwon Hospital, Changwon, Korea. golgy@hanmail.net
- 2Gyeongsang National University School of Medicine, Jinju, Korea.
- 3Gyeongsang Institute of Health Science, Jinju, Korea.
- 4Department of Pathology, Gyeongsang National University Hospital, Jinju, Korea.
- 5Deparment of Otorhinolaryngology, Gyeongsang National University Changwon Hospital, Changwon, Korea.
- 6Department of Surgery, Gyeongsang National University Changwon Hospital, Changwon, Korea.
- KMID: 2403252
- DOI: http://doi.org/10.4132/jptm.2017.07.26
Abstract
- BACKGROUND
The immunotherapeutic role of programmed death-ligand 1 (PD-L1) in life expectancy in many cancers has been highlighted. However, data regarding PD-L1 expression in papillary thyroid carcinoma (PTC) are limited. In this study, we describe the PD-L1 and programmed cell death protein 1 (PD-1) expressions in PTC and analyze their correlation with lymph node (LN) metastasis.
METHODS
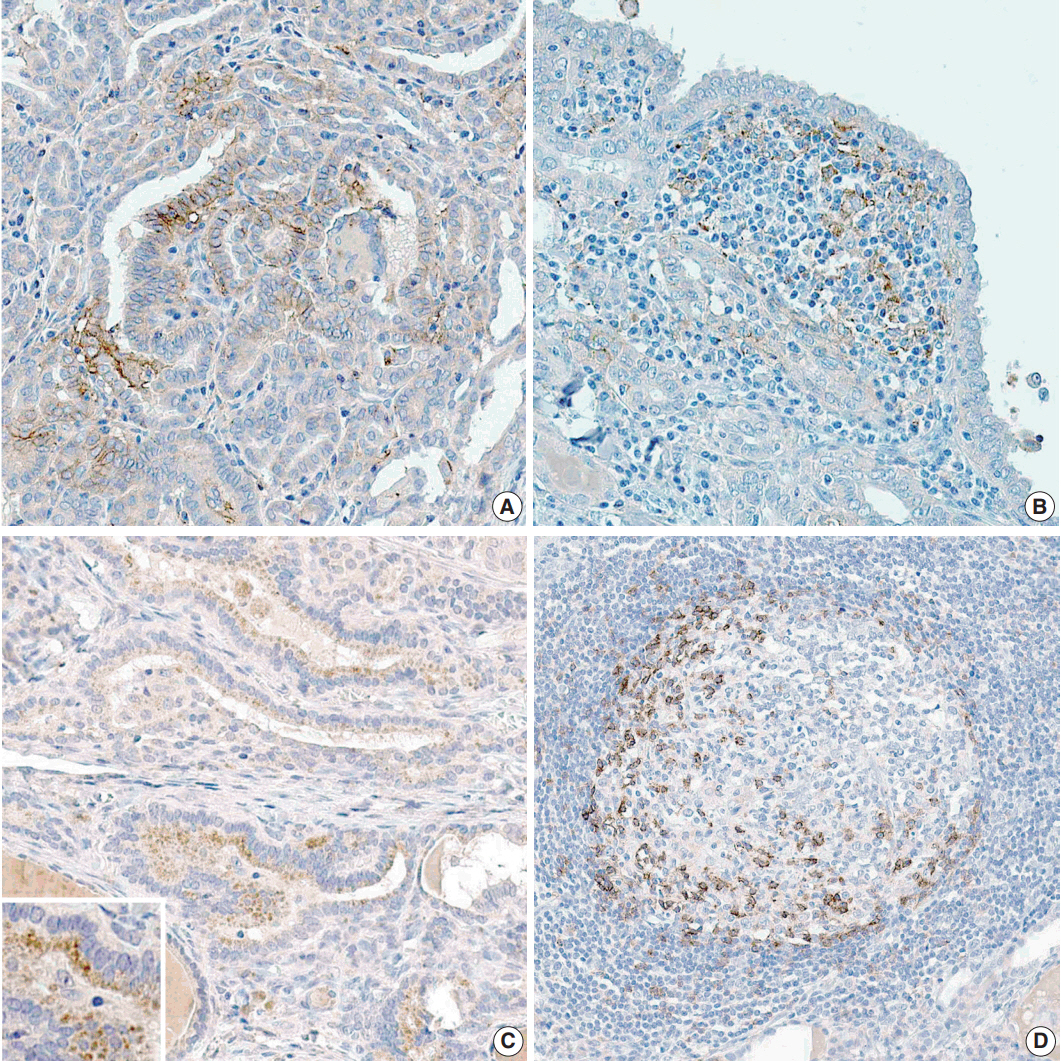
Clinicopathological data were obtained from 116 patients with PTC who were treated in Gyeongsang National University Hospital, Jinju, Korea in 2009. Tissue microarray blocks were made using representative paraffin blocks of classical PTCs excluding follicular variants. Two pathologists graded the proportion and intensity of PD-L1 and PD-1 expression in both tumor and inflammatory cells. According to their proportions, positive PTC cells were scored as negative (0%), grade 1 (1%-50%), and grade 2 (51%-100%). Similarly, positive inflammatory cells were graded as negative (0%), grade 1 (1%-10%), and grade 2 (11%-20%). The intensity of each protein expression was simplified as positive or negative.
RESULTS
A statistically significant correlation exists between the proportions of PD-1 and PD-L1 expression both in papillary carcinoma (p=.001) and peritumoral lymphoid cells in the thyroid (p<.001). In addition, the proportion of PD-L1 expression in PTC cells was closely related to metastatic LNs (p=.036).
CONCLUSIONS
PD-L1 is a valuable predictive marker for LN metastasis in PTC. Immunomodulating therapies that inhibit PD-L1 might be an option for patients with LN metastasis.
MeSH Terms
Figure
Reference
-
1. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016; 387:1540–50.
Article2. Juárez-Salcedo LM, Sandoval-Sus J, Sokol L, Chavez JC, Dalia S. The role of anti-PD-1 and anti-PD-L1 agents in the treatment of diffuse large B-cell lymphoma: The future is now. Crit Rev Oncol Hematol. 2017; 113:52–62.
Article3. Motoshima T, Komohara Y, Ma C, et al. PD-L1 expression in papillary renal cell carcinoma. BMC Urol. 2017; 17:8.
Article4. Fontugne J, Augustin J, Pujals A, et al. PD-L1 expression in perihilar and intrahepatic cholangiocarcinoma. Oncotarget. 2017; 8:24644–51.
Article5. Jiang Y, Lo AW, Wong A, et al. Prognostic significance of tumor-infiltrating immune cells and PD-L1 expression in esophageal squamous cell carcinoma. Oncotarget. 2017; 8:30175–89.
Article6. Ness N, Andersen S, Khanehkenari MR, et al. The prognostic role of immune checkpoint markers programmed cell death protein 1 (PD-1) and programmed death ligand 1 (PD-L1) in a large, multicenter prostate cancer cohort. Oncotarget. 2017; 8:26789–801.
Article7. Park IH, Yang HN, Lee KJ, et al. Tumor-derived IL-18 induces PD-1 expression on immunosuppressive NK cells in triple-negative breast cancer. Oncotarget. 2017; 8:32722–30.
Article8. Jin S, Xu B, Yu L, et al. The PD-1, PD-L1 expression and CD3+ T cell infiltration in relation to outcome in advanced gastric signet-ring cell carcinoma, representing a potential biomarker for immunotherapy. Oncotarget. 2017; 8:38850–62.
Article9. Gravelle P, Burroni B, Péricart S, et al. Mechanisms of PD-1/PD-L1 expression and prognostic relevance in non-Hodgkin lymphoma: a summary of immunohistochemical studies. Oncotarget. 2017; 8:44960–75.
Article10. Zhou Y, Miao J, Wu H, et al. PD-1 and PD-L1 expression in 132 recurrent nasopharyngeal carcinoma: the correlation with anemia and outcomes. Oncotarget. 2017; 8:51210–23.
Article11. Chen N, Fang W, Lin Z, et al. KRAS mutation-induced upregulation of PD-L1 mediates immune escape in human lung adenocarcinoma. Cancer Immunol Immunother. 2017; 66:1175–87.
Article12. Chovanec M, Cierna Z, Miskovska V, et al. Prognostic role of programmed-death ligand 1 (PD-L1) expressing tumor infiltrating lymphocytes in testicular germ cell tumors. Oncotarget. 2017; 8:21794–805.
Article13. Botti G, Collina F, Scognamiglio G, et al. Programmed death ligand 1 (PD-L1) tumor expression is associated with a better prognosis and diabetic disease in triple negative breast cancer patients. Int J Mol Sci. 2017; 18:E459.
Article14. Han J, Hong Y, Lee YS. PD-L1 expression and combined status of PD-L1/PD-1-positive tumor infiltrating mononuclear cell density predict prognosis in glioblastoma patients. J Pathol Transl Med. 2017; 51:40–8.
Article15. Nikiforov YE, Seethala RR, Tallini G, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016; 2:1023–9.16. Nixon IJ, Wang LY, Migliacci JC, et al. An international multi-institutional validation of age 55 years as a cutoff for risk stratification in the AJCC/UICC staging system for well-differentiated thyroid cancer. Thyroid. 2016; 26:373–80.
Article17. Song DH, Ko GH, Lee JH, et al. Myoferlin expression in non-small cell lung cancer: Prognostic role and correlation with VEGFR-2 expression. Oncol Lett. 2016; 11:998–1006.
Article18. Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004; 4:336–47.
Article19. Thompson RH, Dong H, Kwon ED. Implications of B7-H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Cancer Res. 2007; 13(2 Pt 2):709s–15s.
Article20. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012; 12:252–64.
Article21. Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000; 192:1027–34.
Article22. Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002; 8:793–800.
Article23. French JD, Kotnis GR, Said S, et al. Programmed death-1+ T cells and regulatory T cells are enriched in tumor-involved lymph nodes and associated with aggressive features in papillary thyroid cancer. J Clin Endocrinol Metab. 2012; 97:E934–43.
Article24. Zajac AJ, Blattman JN, Murali-Krishna K, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998; 188:2205–13.
Article25. Wherry EJ, Ha SJ, Kaech SM, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007; 27:670–84.
Article26. Bastman JJ, Serracino HS, Zhu Y, et al. Tumor-infiltrating T cells and the PD-1 checkpoint pathway in advanced differentiated and anaplastic thyroid cancer. J Clin Endocrinol Metab. 2016; 101:2863–73.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Retropharyngeal Lymph Node Metastasis of Thyroid Papillary Carcinoma
- A Case of Cystic Lymph Node Metastasis from Thyroid Papillary Microcarcinoma
- Retropharyngeal Lymph Node Metastasis from Thyroid Papillary Carcinoma with Airway Obstruction
- Two Cases of Transoral Resection of Retropharyngeal Lymph Node Metastasis from Papillary Thyroid Carcinoma Diagnosed by PET-CT Follow-Up after Lateral Neck Dissection
- Calcification and Expression of Bone Morphogenetic Protein-4 in Papillary Thyroid Carcinoma