Nat Prod Sci.
2017 Dec;23(4):258-264. 10.20307/nps.2017.23.4.258.
Studies on the Chemical Constituents from the Seeds of Zizyphus jujuba var. inermis
- Affiliations
-
- 1R&D Headquarters, Korea Ginseng Corp., Daejeon 34128, Republic of Korea. leenamkyung@kgc.co.kr
- KMID: 2401577
- DOI: http://doi.org/10.20307/nps.2017.23.4.258
Abstract
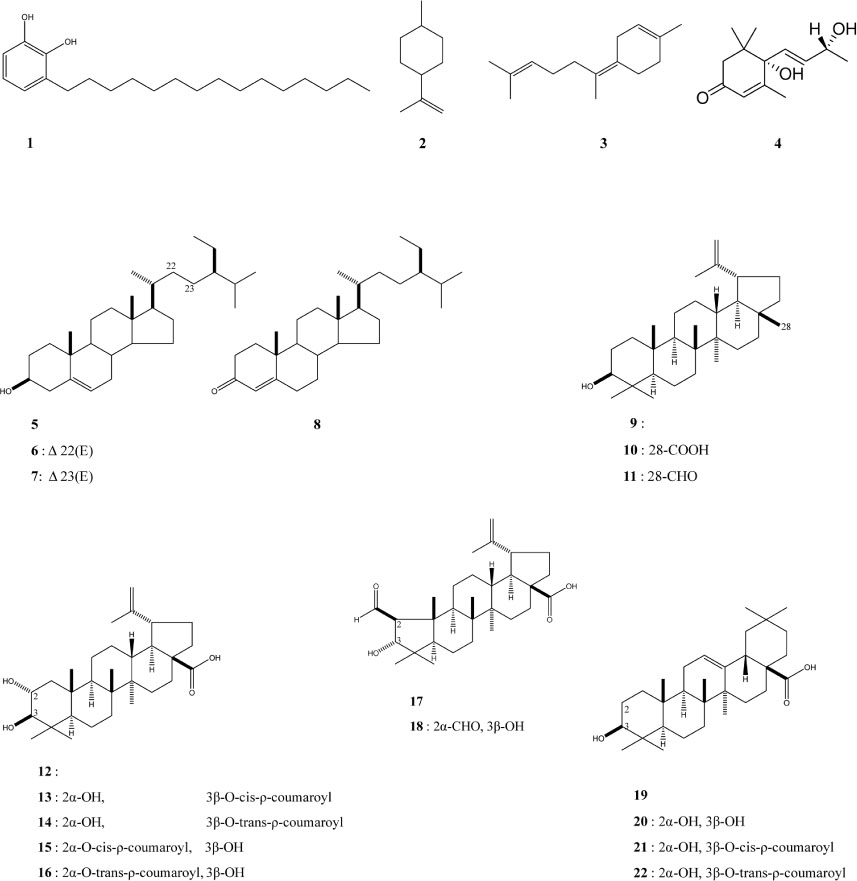
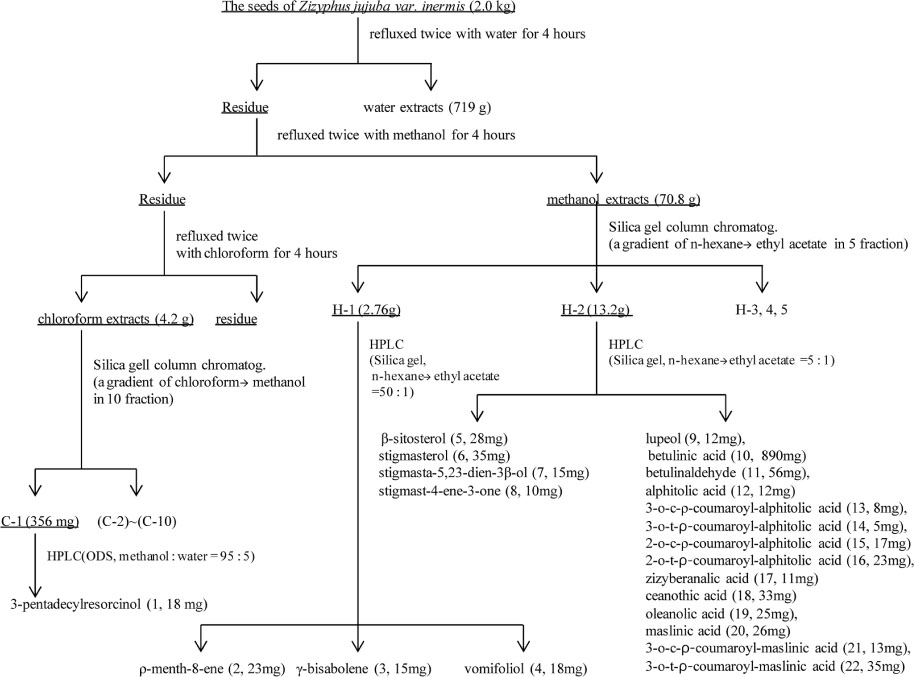
- This study analyzed the seeds of Zizyphus jujuba var. inermis commonly used as a remedy in traditional Chinese medicine, in order to determine its various biologically active compounds. Through process 3-pentadecylcatechol, Ï-menth-8-ene, and γ-bisabolene were isolated and identified for the first time which are urushiol, monoterpenoidal, and sesquiterpenoidal compounds, respectively. Also, found were another sesquiterpenoidal compounds, vomifoliol, and four steroidal compounds, β-sitosterol, stigmasterol, stigmasta-5,23-dien-3β-ol, and stigmast-4-en-3-one. In addition, fourteen triterpenoidal compounds were isolated and identified. These were lupeol, betulinic acid, betulinaldehyde, alphitolic acid, 3-O-cis-Ï-coumaroyl-alphitolic acid, 3-O-trans-Ï-coumaroylalphitolic acid, 2-O-cis-Ï-coumaroyl-alphitolic acid, 2-O-trans-Ï-coumaroyl-alphitolic acid, zizyberanalic acid, ceanothic acid, oleanolic acid, maslinic acid, 3-O-cis-Ï-coumaroyl-maslinic acid, and 3-O-trans-Ï-coumaroylmaslinic acid. The structures were identified by comparing of the spectroscopic experiments, NMR and MS, and then compared that reported data, respectively. Three extracts of water, methanol, and chloroform from the seeds showed a weak anti-proliferative effect, anti-microbial activity, and anti-oxidant effect, respectively.
Keyword
MeSH Terms
Figure
Reference
-
1. Greca MD, Monaco P, Previtera L. J Nat Pro. 1990; 53:1430–1435.2. Yagi A, Okamura N, Haraguchi Y, Noda K, Nishioka I. Chem Pharm Bull. 1978; 26:1798–1802.3. ElSohly MA, Adawadkar PD, Ma CY, Turner CE. J Nat Prod. 1982; 45:532–538.4. Tilton B. Wilderness First Responder: How to Recognize, Treat, and Prevent Emergencies in the Backcountry. USA: Globe Pequot;2004. p. 320–320.5. Kim MJ, Choi YH, Kim WG, Kwak SS. Korean J Plant Resour. 1997; 10:227.6. Kim MJ, Choi WC, Barshinikov AM, Kobayashi A. Korean J Med Crop Sci. 2002; 10:288–230.7. Kim JC, Ahn JK, Ko SY, Choi YH, Kim DH, Lee TY. Clean Tech. 2007; 13:22–27.8. Bazyl'chik VV, Ryabushkin NM, Staninets VI, Shingel I. Zh Org Khim. 1978; 14:2280–2286.9. Wolinsky LE, Faulkner DJ, Finer J, Clardy J. J Org Chem. 1976; 41:697–699.10. Siddiqui BS, Kardar MN, Ali ST, Khan S. Helv Chim Acta. 2003; 86:2164–2169.11. Itoh T, Sica D, Djerassi C. J Org Chem. 1983; 48:890–892.12. Sofowora EA, Hardman R. Phytochemistry. 1973; 12:403–406.13. Sholichin M, Yamasaki K, Kasai R, Tanaka O. Chem Pharm Bull. 1980; 28:1006–1008.14. Ibrahim SR, Fouad MA, Abdel-Lateff A, Okino T, Mohamed GA. Nat Prod Res. 2014; 28:1765–1771.15. Quiroz S, Cespedes CL, Alderete JB, Alarcon J. Indust Crop Prod. 2015; 74:759–766.16. Lee SS, Lin CJ, Liu KC. J Nat Prod. 1992; 55:602–606.17. Yagi A, Okamura N, Haraguchi Y, Noda K, Nishioka I. Chem Pharm Bull. 1978; 26:3075–3079.18. Lee SM, Min BS, Lee CG, Kim KS, Kho YH. Planta Med. 2003; 69:1051–1054.19. Lee S, Yoon HJ, Choi SY, Moon MJ, Jin SY, Yoon YH. J Food Hyg Saf. 2013; 28:241–246.20. Kim HK, Joo KJ. J Korean Soc Food Sci Nutr. 2005; 34:750–754.21. Jou YJ, Chen CJ, Liu YC, Way TD, Lai CH, Hua CH, Wang CY, Huang SH, Kao JY, Lin CW. Proteomics. 2015; 15:3296–3309.22. Nair JJ, Mulaudzi RB, Chukwujekwu JC, Van Heerden FR, Van Staden J. South Afric J Bot. 2013; 86:111–115.23. Jeong SY, Zhao BT, Kim YH, Min BS, Woo MH. Nat Prod Sci. 2013; 19:366–371.24. Talabani NS, Tofiq DI. Int J Med Arom Plants. 2012; 2:536–539.25. Yu HH, Moon HD, Hwang JY, Kim SY, Jeong SI, Jeon BH, You YO. Korean J Orient Physiol Pathol. 2007; 21:70–75.26. Scholtysek C, Krukiewicz AA, Alonso JL, Sharma KP, Sharma PC, Goldmann WH. Biochem Biophys Res Commun. 2009; 379:795–798.27. Liu D, Dai C, Xu D. Bioinformatics and Biomedicine Workshps (BIBMW). In : IEEE Intrnational Conference; 2011. p. 440–445.28. Fomogne-Fodjo MC, Ndinteh DT, Olivier DK, Kempgens P, van Vuuren S, Krause RW. J Ethnopharmacol. 2017; 195:238–245.29. Ghosh S, Mukhopadhyay S, Sarkar M, Mandal A, Das V, Kumar A, Giri B. Chem Biol Interact. 2017; 268:68–76.30. Morrison SA, Li H, Webster D, Johnson JA, Gray CA. J Ethnopharmacol. 2016; 188:200–203.31. Gossan DP, Alabdul Magid A, Yao-Kouassi PA, Ahibo Coffy A, Josse J, Gangloff SC, Morjani H, Voutquenne-Nazabadioko L. Phytochemistry. 2017; 134:71–77.32. Bai LY, Chiu CF, Chiu SJ, Chen YW, Hu JL, Wu CY, Weng JR. J Funct Foods. 2015; 18:368–378.33. Li XC, Cai L, Wu CD. Phytochem. 1997; 46:97–102.34. Rufino-Palomares EE, Reyes-Zurita FJ, Garcia-Salguero L, Mokhtari K, Medina PP, Lupianez JA, Peragon J. J Proteomics. 2013; 83:15–25.35. An B, Lee CG, Song MK, Ryu JC, Lee S, Park SJ, Zhao D, Kim SB, Park C, Lee SH, Hong SW, Choi JW. . React Funct Polymer. 2015; 93:138–147.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Blockade of vascular angiogenesis by Aspergillus usamii var. shirousamii-transformed Angelicae Gigantis Radix and Zizyphus jujuba
- Ethanolic Extract of the Seed of Zizyphus jujuba var. spinosa Ameliorates Cognitive Impairment Induced by Cholinergic Blockade in Mice
- Chemical Constituents of Nelumbo nucifera Seeds
- Anti-Influenza Activity of Betulinic Acid from Zizyphus jujuba on Influenza A/PR/8 Virus
- Spinosin, a C-Glucosylflavone, from Zizyphus jujuba var. spinosa Ameliorates Abeta1-42 Oligomer-Induced Memory Impairment in Mice