Nat Prod Sci.
2017 Dec;23(4):253-257. 10.20307/nps.2017.23.4.253.
Chemical Constituents of Nelumbo nucifera Seeds
- Affiliations
-
- 1College of Pharmacy, The Catholic University of Korea, Bucheon 14662, Korea. kdyoon@catholic.ac.kr
- KMID: 2401576
- DOI: http://doi.org/10.20307/nps.2017.23.4.253
Abstract
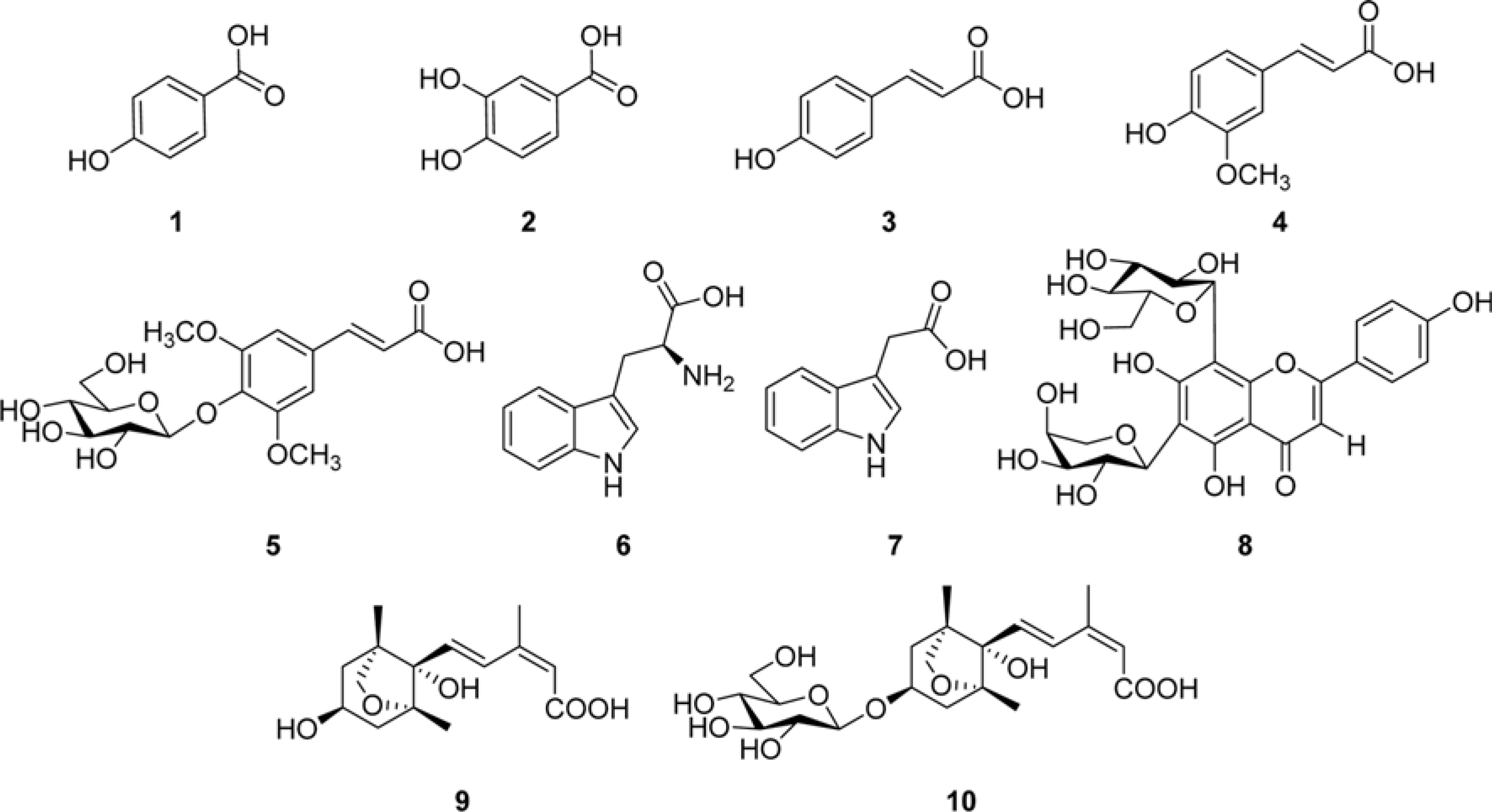
- The phytochemical study for the extract of Nelumbo nucifera (Nymphaceae) seeds has led to the isolation of ten compounds including five simple phenolic compounds, two indole derivatives, a flavonoid glycoside, two abscisic acid derivatives. The interpretation of 1D and 2D NMR and ESI-Q-TOF-MS spectroscopic data revealed the chemical structures of isolates to be p-hydroxybenzoic acid (1), protocatechuic acid (2), (E)-p-coumaric acid (3), (E)-ferulic acid (4), (E)-sinapate-4-O-β-D-glucopyranoside (5), tryptophan (6), 3-indoleacetic acid (7), isoschaftoside (8), dihydrophaseic acid (9), dihydrophaseic acid 3"²-O-β-D-glucopyranoside (10). To the best of our knowledge, 1 - 5 and 7 were identified for the first time from N. nucifera seeds, and the presence of dihydrophaseic acid (9) and its glucoside (10) were demonstrated secondly in this plant.
Keyword
Figure
Reference
-
References
(1). The Compilation Committee of Pharmacognosy Textbook. Pharmacognosy; Dong-Myeung press: Korea,. 2015. 358–360.(2). Sharma B. R.., Gautam L. N.., Adhikari D.., Karki R.Phytother. Res. 2017. 31:3–26.
Article(3). Dhakal R. C.., Rajbhandari M.., Kalauni S. K.., Awale S.., Gewali M. B. J.Nepal Chem. Soc. 2008/2009. 23:89–92.(4). Flamini G.., Antognoli E.., Morelli I.Phytochemistry. 2001. 57:559–564.(5). Nguyen D. H.., Zhao B. T.., Le D. D.., Kim K. Y.., Kim Y. H.., Yoon Y. H.., Ko J. Y.., Woo K. S.., Woo M. H.Nat. Prod. Sci. 2016. 22:140–145.(6). Woo K. W.., Lee K. R.Nat. Prod. Sci. 2013. 19:221–226.(7). Wolfram K.., Schmidt J.., Wray V.., Milkowski C.., Schliemann W.., Strack D.Phytochemistry. 2010. 71:1076–1084.(8). Kim C. S.., Kim K. H.., Lee K. R.Nat. Prod. Sci. 2014. 20:86–90.(9). Xie C.., Veitch N. C.., Houghton P. J.., Simmonds M. S.Chem. Pharm. Bull. 2003. 51:1204–1207.(10). Seo J. H.., Choi Y. H.., Yoo M. Y.., Hong K. S.., Lee B. H.., Yon G. H.., Kim Y. S.., Kim Y. K.., Ryu S. Y.Kor. J. Pharmacogn. 2006. 37:290–297.(11). Youn U. J.., Lee J.., Nam J. W.., Lee Y. J.., Seo E. K.Bull. Korean Chem. Soc. 2011. 32:4083–4085.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficient Isolation of Dihydrophaseic acid 3′-O-β-D-Glucopyranoside from Nelumbo nucifera Seeds Using High-performance Countercurrent Chromatography and Reverse-phased High-performance Liquid Chromatography
- Antioxidant Activity and Phenolic Content of Different Parts of Lotus and Optimization of Extraction Condition using Response Surface Methodology
- Quality Characteristics and Optimal Conditions for Sweet Rice Muffin Lotus (Nelumbo nucifera Gaertn) Seed Powder, Applying the Response Surface Method
- Nelumbo nucifera Leaf Extract Regulates Lipid Metabolism and Differentiation in 3T3-L1 Adipocytes and db/db Mice
- Chemical Use and Associated Health Concerns in the Semiconductor Manufacturing Industry