Pediatr Infect Vaccine.
2017 Dec;24(3):125-133. 10.14776/piv.2017.24.3.125.
Evaluation of Antibodies Against Haemophilus influenzae Type b in Korean Adults
- Affiliations
-
- 1Department of Pediatrics, Medical Research Institute, Ewha Womans University School of Medicine, Seoul, the Republic of Korea. kaykim@ewha.ac.kr
- 2Center for Vaccine Evaluation and Study, Medical Research Institute, Ewha Womans University School of Medicine, Seoul, the Republic of Korea.
- KMID: 2401386
- DOI: http://doi.org/10.14776/piv.2017.24.3.125
Abstract
- PURPOSE
After the introduction of Haemophilus influenzae type b (Hib) vaccine in 1995 in Korea, it was included in the national immunization program in 2013. In the post-Hib vaccine era, some studies in other countries reported that invasive Hib disease affects adults, especially the elderly and immunocompromised persons, more often than it affects children. To evaluate disease susceptibility, quantitative and qualitative analysis of anti-polyribosylribitol phosphate (PRP) antibodies were carried out in Korean adults aged 20 to 85 years.
METHODS
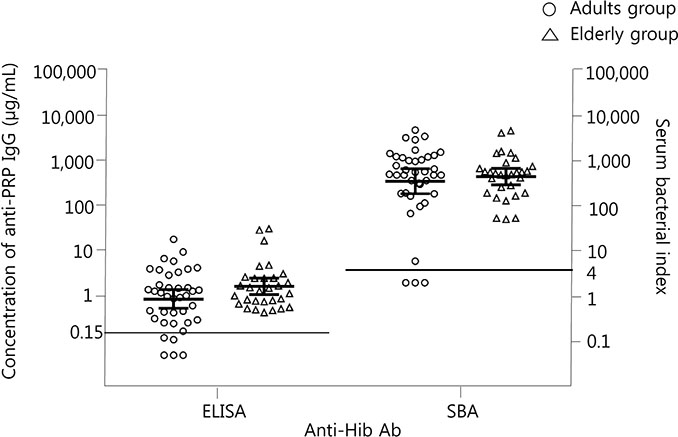
Sera were collected from 39 healthy adults (20 to 50 years of age) and from 30 elderly adults (75 to 85 years of age) who did not have immune-compromising conditions. The concentration of anti-PRP immunoglobulin G (IgG) and serum bactericidal indices (SBIs) were measured by enzyme-linked immunosorbent assay and serum bactericidal assay.
RESULTS
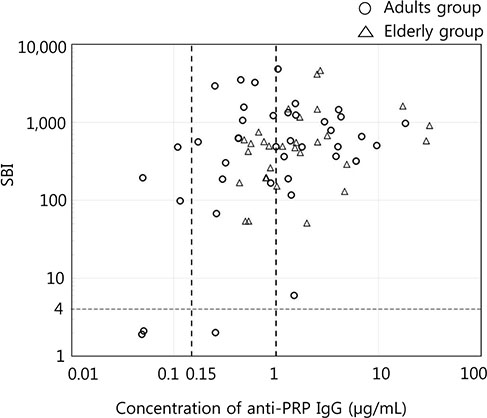
Geometric mean concentrations of anti-PRP IgG and geometric mean SBIs were 0.88 µg/mL (95% confidence interval [CI], 0.17 to 3.85) and 354 (95% CI, 50 to 2,499) in young adults and 1.67 µg/mL (95% CI, 0.53 to 5.24) and 449 (95% CI, 146 to 1,376) in elderly adults, respectively. When the threshold of seropositivity for anti-PRP IgG was applied as 0.15 or 1.0 µg/mL, which is the protective antibody level in children, seropositive rates were 87.2% or 53.8% in young adults and 100% or 60% in elderly adults. The seropositivity rates of the SBI (SBI ≥4) were 82.1% and 100% in the groups, respectively.
CONCLUSIONS
Most subjects in the adult and elderly adult groups display immunity to Hib based on quantitative and qualitative antibody levels, but not all. Because high immunization and low Hib circulation rates may reduce the natural Hib immunity in the population, monitoring Hib immunity as well as disease are needed continuously.
Keyword
MeSH Terms
Figure
Reference
-
1. Chandran A, Watt JP, Santosham M. Haemophilus influenzae vaccine. In : Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 6th ed. Philadelphia: Elsevier Health Sciences;2013. p. 167–182.2. Pittman M. Variation and type specificity in the bacterial species Hemophilus influenzae. J Exp Med. 1931; 53:471–492.
Article3. Kim KH, Lee H, Chung EH, Kang JH, Kim JH, Kim JS, et al. Immunogenicity and safety of two different Haemophilus influenzae type b conjugate vaccines in Korean infants. J Korean Med Sci. 2008; 23:929–936.
Article4. Kim HW, Kim KH, Kim J, Nahm MH. A high throughput serum bactericidal assay for antibodies to Haemophilus influenzae type b. BMC Infect Dis. 2016; 16:473.
Article5. Mackenzie GA, Ikumapayi UN, Scott S, Idoko O, Odutola A, Ndiaye M, et al. Increased disease due to Haemophilus influenzae type b: population-based surveillance in eastern Gambia, 2008–2013. Pediatr Infect Dis J. 2015; 34:e107–e112.6. Idoko OT, Roberts E, Cox M, Jafali J, Njie-Jobe J, Mackenzie G, et al. Antibodies against Haemophilus influenzae type b in The Gambia: investigating the extent of protection across age groups. Vaccine. 2014; 32:4620–4624.
Article7. McVernon J, Trotter CL, Slack MP, Ramsay ME. Trends in Haemophilus influenzae type b infections in adults in England and Wales: surveillance study. BMJ. 2004; 329:655–658.
Article8. Dworkin MS, Park L, Borchardt SM. The changing epidemiology of invasive Haemophilus influenzae disease, especially in persons > or = 65 years old. Clin Infect Dis. 2007; 44:810–816.
Article9. Rubach MP, Bender JM, Mottice S, Hanson K, Weng HY, Korgenski K, et al. Increasing incidence of invasive Haemophilus influenzae disease in adults, Utah, USA. Emerg Infect Dis. 2011; 17:1645–1650.10. Shuel M, Hoang L, Law DK, Tsang R. Invasive Haemophilus influenzae in British Columbia: non-Hib and non-typeable strains causing disease in children and adults. Int J Infect Dis. 2011; 15:e167–e173.
Article11. Kim KH, Lim SY. Validation of enzyme immunoassay for the quantitative measurement of human IgG antibodies specific for Haemophilus influenzae type b capsular polysaccharide. Korean J Pediatr. 2007; 50:143–150.
Article12. Peltola H, Kayhty H, Sivonen A, Makela H. Haemophilus influenzae type b capsular polysaccharide vaccine in children: a double-blind field study of 100,000 vaccinees 3 months to 5 years of age in Finland. Pediatrics. 1977; 60:730–737.
Article13. Oh SY, Griffiths D, John T, Lee YC, Yu LM, McCarthy N, et al. School-aged children: a reservoir for continued circulation of Haemophilus influenzae type b in the United Kingdom. J Infect Dis. 2008; 197:1275–1281.
Article14. Kayhty H, Peltola H, Karanko V, Makela PH. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis. 1983; 147:1100.
Article15. Nix EB, Hawdon N, Gravelle S, Biman B, Brigden M, Malik S, et al. Risk of invasive Haemophilus influenzae type b (Hib) disease in adults with secondary immunodeficiency in the post-Hib vaccine era. Clin Vaccine Immunol. 2012; 19:766–771.
Article16. Chalmers C. Necrotising fasciitis complicating Haemophilus influenzae type b epiglottitis in an adult. J Laryngol Otol. 2010; 124:807–809.
Article17. Saito T, Matsunaga H, Matsumura Y, Segawa H, Takakura S, Nagao M, et al. Necrotizing fasciitis caused by Haemophilus influenzae type b in an elderly patient. J Clin Microbiol. 2009; 47:852–854.
Article18. Chew CG, Clarkson AR, Faull RJ. Relapsing CAPD peritonitis with rapid peritoneal sclerosis due to Haemophilus influenzae. Nephrol Dial Transplant. 1997; 12:821–822.
Article19. Neuhaus TJ, Iselin H, Nadal D. Haemophilus influenzae: a cause of peritonitis in peritoneal dialysis. Nephrol Dial Transplant. 1996; 11:199–200.
Article20. Public Health Agency of Canada. Canadian immunization guide 2006. 7th ed. Ottawa: Public Health Agency of Canada;2006.21. Collins S, Ramsay M, Campbell H, Slack MP, Ladhani SN. Invasive Haemophilus influenzae type b disease in England and Wales: who is at risk after 2 decades of routine childhood vaccination? Clin Infect Dis. 2013; 57:1715–1721.
Article22. Skoff TH, Farley MM, Petit S, Craig AS, Schaffner W, Gershman K, et al. Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990–2007. Clin Infect Dis. 2009; 49:85–92.
Article23. Edwards MS, Baker CJ. Group B streptococcal infections in elderly adults. Clin Infect Dis. 2005; 41:839–847.
Article24. Jelonek MT, Chang SJ, Chiu CY, Park MK, Nahm MH, Ward JI. Comparison of naturally acquired and vaccine-induced antibodies to Haemophilus influenzae type b capsular polysaccharide. Infect Immun. 1993; 61:5345–5350.
Article25. Kim YJ, Hwang JY, Choi SH, Kong E, Kim Y, Park KS, et al. Antibody responses in hematopoietic cell transplantation recipients after vaccination against Haemophilus influenzae type b and Streptococcus pneumoniae. Korean J Pediatr Infect Dis. 2014; 21:81–95.
Article26. Lee H, Nahm MH, Burton R, Kim KH. Immune response in infants to the heptavalent pneumococcal conjugate vaccine against vaccine-related serotypes 6A and 19A. Clin Vaccine Immunol. 2009; 16:376–381.
Article27. Song JY, Moseley MA, Burton RL, Nahm MH. Pneumococcal vaccine and opsonic pneumococcal antibody. J Infect Chemother. 2013; 19:412–425.
Article28. World Health Organization. Guidelines on clinical evaluation of vaccines: regulatory expectations. Proposed revision of WHO TRS 924, Annex 1 [Internet]. Geneva: World Health Organization;2004. cited 2017 Nov 7. Available from: http://www.who.int/biologicals/BS2287_Clinical_guidelines_final_LINE_NOs_20_July_2016.pdf.29. Cho HK, Park IH, Burton RL, Kim KH. Impact of IgM antibodies on cross-protection against pneumococcal serogroups 6 and 19 after immunization with 7-valent pneumococcal conjugate vaccine in children. J Korean Med Sci. 2016; 31:950–956.
Article30. Kim KH. Present status and prospects of Haemophilus influenzae type b (Hib) immunization. Korean J Pediatr. 2006; 49:242–250.
Article31. Shinefield HR, Black S. Postlicensure surveillance for Haemophilus influenzae type b invasive disease after use of Haemophilus influenzae type b oligosaccharide CRM197 conjugate vaccine in a large defined United States population: a four-year eight-month follow-up. Pediatr Infect Dis J. 1995; 14:978–981.
Article32. Watt JP, Levine OS, Santosham M. Global reduction of Hib disease: what are the next steps? Proceedings of the meeting Scottsdale, Arizona, September 22–25, 2002. J Pediatr. 2003; 143:6 Suppl. S163–S187.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Neurologic Sequelae and a Case of Peripheral Gangrene of Extremities Associated with Haemophilus influenzae Type b Meningitis
- Natural Anti-PRP Antibody Levels ot Haemophilus Influenzae Type b(Hib) and Changs of Antibody Levels after Three Doses of Vacination
- Haemophilus influenzae vaccine
- Cerebral Venous Sinus Thrombosis with Meningitis and Septicemia due to Haemophilus influenzae Type f in an Immunocompetent Child
- Validation of enzyme immunoassay for the quantitative measurement of human IgG antibodies specific for Haemophilus influenzae Type b capsular polysaccharide