J Clin Neurol.
2018 Jan;14(1):90-97. 10.3988/jcn.2018.14.1.90.
Diagnosis of Early-Stage Idiopathic Parkinson's Disease Using High-Resolution Quantitative Susceptibility Mapping Combined with Histogram Analysis in the Substantia Nigra at 3 T
- Affiliations
-
- 1Department of Radiology, Gachon University Gil Medical Center, Incheon, Korea.
- 2Department of Neurology, Gachon University Gil Medical Center, Incheon, Korea.
- 3Department of Electrical and Computer Engineering, Seoul National University, Seoul, Korea. jonghoyi@snu.ac.kr
- 4Department of Radiology, Seoul St. Mary's Hospital, Seoul, Korea. yhnam83@gmail.com
- KMID: 2399604
- DOI: http://doi.org/10.3988/jcn.2018.14.1.90
Abstract
- BACKGROUND AND PURPOSE
To test whether nigrosome-1 imaging using high-resolution quantitative susceptibility mapping (QSM) combined with histogram analysis can improve the diagnostic accuracy in early-stage idiopathic Parkinson's disease (IPD) patients.
METHODS
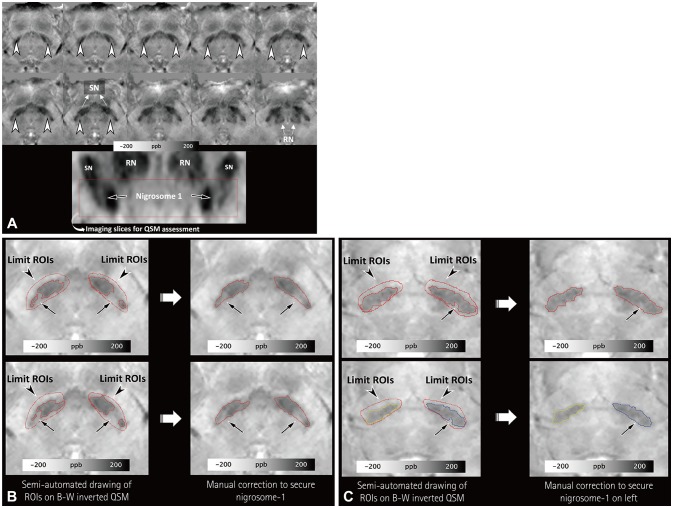
Three-dimensional multiecho gradient-recalled echo images (0.5×0.5×1.0 mm³) were obtained at 3 T for QSM in 38 patients with IPD and 25 healthy subjects. To segment the substantia nigra (SN), regions of interest (ROIs) were semiautomatically drawn at the location below the red nucleus, and the normal-appearing nigrosome-1 was determined by manual correction. QSM histograms were obtained within the ROI. The segmented SN regions on the right and left that had higher mean susceptibility values and fewer voxels with susceptibility values lower than 60, 65, 70, 75, and 80 ppb were chosen for comparisons between the IPD patients and healthy subjects. These results were compared with those of the visual assessments of nigrosome-1 in susceptibility map-weighted imaging (SMWI) by analyzing receiver operating characteristics curves.
RESULTS
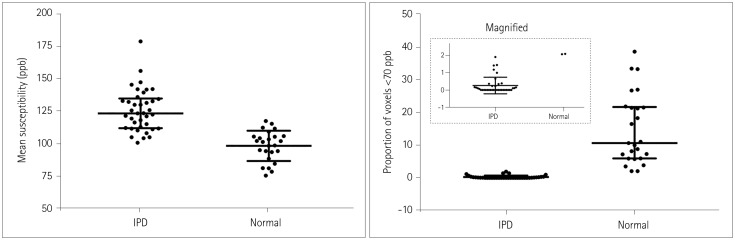
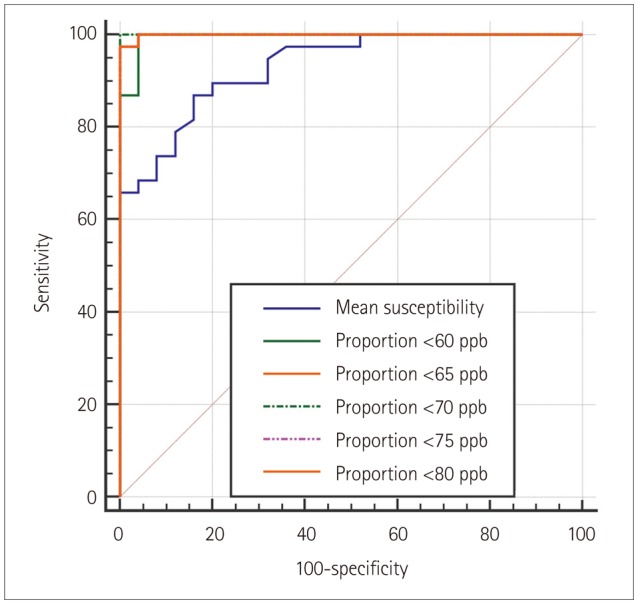
The proportion of voxels with susceptibility values lower than 70 ppb showed the best diagnostic performance, with its value differing significantly between the IPD patients (median=0, interquartile range=0-0.23) and healthy subjects (median=10.67, interquartile range=5.98-21.57) (p < 0.0001). The number of voxels with susceptibility values lower than 60, 65, 70, 75, and 80 ppb showed worse diagnostic performances but were still significantly better than that of the mean susceptibility value (p=0.0249, 0.0192, 0.0183, 0.0191, and 0.0186, respectively), which also differed significantly between the two groups: 125.81±16.27 ppb (mean±standard deviation) in IPD versus 98.41±11.70 ppb in healthy subjects (p < 0.0001). Additionally, using the proportion of voxels with susceptibility values lower than 70 ppb provided significantly better diagnostic performance than did visual assessments of SMWI (p=0.0143).
CONCLUSIONS
High-spatial-resolution QSM combined with histogram analysis at 3 T can improve the diagnostic accuracy of early-stage IPD.
MeSH Terms
Figure
Reference
-
1. Blazejewska AI, Schwarz ST, Pitiot A, Stephenson MC, Lowe J, Bajaj N, et al. Visualization of nigrosome 1 and its loss in PD: pathoanatomical correlation and in vivo 7 T MRI. Neurology. 2013; 81:534–540. PMID: 23843466.
Article2. Schwarz ST, Afzal M, Morgan PS, Bajaj N, Gowland PA, Auer DP. The ‘swallow tail’ appearance of the healthy nigrosome-a new accurate test of Parkinson’s disease: a case-control and retrospective cross-sectional MRI study at 3T. PLoS One. 2014; 9:e93814. PMID: 24710392.3. Cosottini M, Frosini D, Pesaresi I, Costagli M, Biagi L, Ceravolo R, et al. MR imaging of the substantia nigra at 7 T enables diagnosis of Parkinson disease. Radiology. 2014; 271:831–838. PMID: 24601752.
Article4. Cosottini M, Frosini D, Pesaresi I, Donatelli G, Cecchi P, Costagli M, et al. Comparison of 3T and 7T susceptibility-weighted angiography of the substantia nigra in diagnosing Parkinson disease. AJNR Am J Neuroradiol. 2015; 36:461–466. PMID: 25376811.
Article5. Noh Y, Sung YH, Lee J, Kim EY. Nigrosome 1 detection at 3T MRI for the diagnosis of early-stage idiopathic Parkinson disease: assessment of diagnostic accuracy and agreement on imaging asymmetry and clinical laterality. AJNR Am J Neuroradiol. 2015; 36:2010–2016. PMID: 26294646.
Article6. Sung YH, Noh Y, Lee J, Kim EY. Drug-induced Parkinsonism versus idiopathic Parkinson disease: utility of nigrosome 1 with 3-T imaging. Radiology. 2016; 279:849–858. PMID: 26690908.
Article7. Deh K, Nguyen TD, Eskreis-Winkler S, Prince MR, Spincemaille P, Gauthier S, et al. Reproducibility of quantitative susceptibility mapping in the brain at two field strengths from two vendors. J Magn Reson Imaging. 2015; 42:1592–1600. PMID: 25960320.
Article8. Lin PY, Chao TC, Wu ML. Quantitative susceptibility mapping of human brain at 3T: a multisite reproducibility study. AJNR Am J Neuroradiol. 2015; 36:467–474. PMID: 25339652.
Article9. Barbosa JH, Santos AC, Tumas V, Liu M, Zheng W, Haacke EM, et al. Quantifying brain iron deposition in patients with Parkinson's disease using quantitative susceptibility mapping, R2 and R2*. Magn Reson Imaging. 2015; 33:559–565. PMID: 25721997.10. He N, Ling H, Ding B, Huang J, Zhang Y, Zhang Z, et al. Region-specific disturbed iron distribution in early idiopathic Parkinson's disease measured by quantitative susceptibility mapping. Hum Brain Mapp. 2015; 36:4407–4420. PMID: 26249218.
Article11. Murakami Y, Kakeda S, Watanabe K, Ueda I, Ogasawara A, Moriya J, et al. Usefulness of quantitative susceptibility mapping for the diagnosis of Parkinson disease. AJNR Am J Neuroradiol. 2015; 36:1102–1108. PMID: 25767187.
Article12. Acosta-Cabronero J, Cardenas-Blanco A, Betts MJ, Butryn M, Valdes-Herrera JP, Galazky I, et al. The whole-brain pattern of magnetic susceptibility perturbations in Parkinson's disease. Brain. 2017; 140:118–131. PMID: 27836833.
Article13. Azuma M, Hirai T, Yamada K, Yamashita S, Ando Y, Tateishi M, et al. Lateral asymmetry and spatial difference of iron deposition in the substantia nigra of patients with Parkinson disease measured with quantitative susceptibility mapping. AJNR Am J Neuroradiol. 2016; 37:782–788. PMID: 26822728.
Article14. Du G, Liu T, Lewis MM, Kong L, Wang Y, Connor J, et al. Quantitative susceptibility mapping of the midbrain in Parkinson's disease. Mov Disord. 2016; 31:317–324. PMID: 26362242.
Article15. Langkammer C, Pirpamer L, Seiler S, Deistung A, Schweser F, Franthal S, et al. Quantitative susceptibility mapping in Parkinson's disease. PLoS One. 2016; 11:e0162460. PMID: 27598250.
Article16. Guan X, Xuan M, Gu Q, Huang P, Liu C, Wang N, et al. Regionally progressive accumulation of iron in Parkinson's disease as measured by quantitative susceptibility mapping. NMR Biomed. 2017; 30:e3489.
Article17. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992; 55:181–184. PMID: 1564476.
Article18. Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology. 1967; 17:427–442. PMID: 6067254.
Article19. Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. I. Nigrosomes and the nigral matrix, a compartmental organization based on calbindin D(28K) immunohistochemistry. Brain. 1999; 122:1421–1436. PMID: 10430829.20. Massey LA, Miranda MA, Al-Helli O, Parkes HG, Thornton JS, So PW, et al. 9.4 T MR microscopy of the substantia nigra with pathological validation in controls and disease. Neuroimage Clin. 2016; 13:154–163. PMID: 27981030.
Article21. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988; 44:837–845. PMID: 3203132.
Article22. Wang N, Yang H, Li C, Fan G, Luo X. Using 'swallow-tail' sign and putaminal hypointensity as biomarkers to distinguish multiple system atrophy from idiopathic Parkinson's disease: a susceptibility-weighted imaging study. Eur Radiol. 2017; 27:3174–3180. PMID: 28105503.
Article23. Nam Y, Gho SM, Kim DH, Kim EY, Lee J. Imaging of nigrosome 1 in substantia nigra at 3T using multiecho susceptibility map-weighted imaging (SMWI). J Magn Reson Imaging. 2017; 46:528–536. PMID: 27859983.
Article24. Bae YJ, Kim JM, Kim E, Lee KM, Kang SY, Park HS, et al. Loss of Nigral Hyperintensity on 3 Tesla MRI of Parkinsonism: comparison with (123) I-FP-CIT SPECT. Mov Disord. 2016; 31:684–692. PMID: 26990970.25. Reiter E, Mueller C, Pinter B, Krismer F, Scherfler C, Esterhammer R, et al. Dorsolateral nigral hyperintensity on 3.0T susceptibility-weighted imaging in neurodegenerative Parkinsonism. Mov Disord. 2015; 30:1068–1076. PMID: 25773707.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prospect of cell therapy for Parkinson's disease
- Convolutional Neural Network-Based Automatic Segmentation of Substantia Nigra on Nigrosome and Neuromelanin Sensitive MR Images
- Non-Motor Symptom Burdens Are Not Associated with Iron Accumulation in Early Parkinson's Disease: a Quantitative Susceptibility Mapping Study
- Genetic Basis of Parkinson Disease
- Imaging of Dopaminergic System in Movement Disorders