J Korean Assoc Oral Maxillofac Surg.
2017 Dec;43(6):373-387. 10.5125/jkaoms.2017.43.6.373.
Stepwise verification of bone regeneration using recombinant human bone morphogenetic protein-2 in rat fibula model
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, Yonsei University College of Dentistry, Seoul, Korea. kimoms@yuhs.ac
- 2Department of Oral and Maxillofacial Surgery, Wonkwang University Sanbon Hospital, Gunpo, Korea.
- 3Oral Cancer Research Institute, Yonsei University College of Dentistry, Seoul, Korea.
- 4Research Institute for Dental Biomaterials & Bioengineering, Yonsei University College of Dentistry, Seoul, Korea.
- KMID: 2398992
- DOI: http://doi.org/10.5125/jkaoms.2017.43.6.373
Abstract
OBJECTIVES
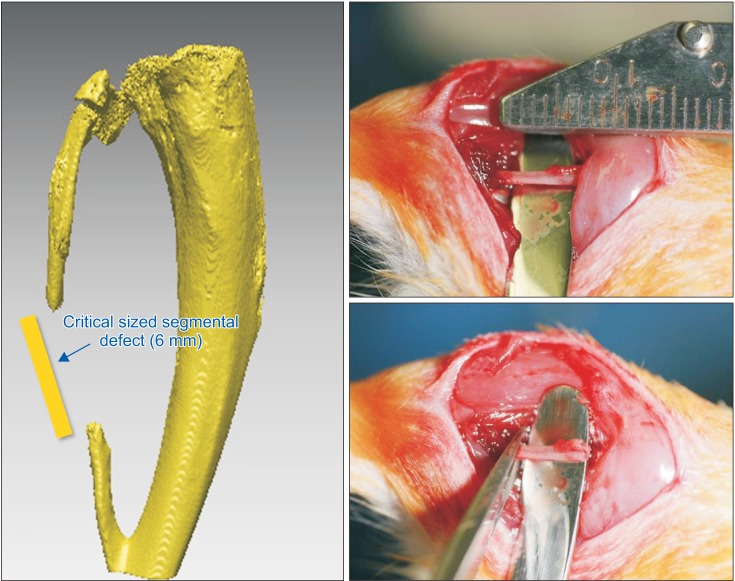
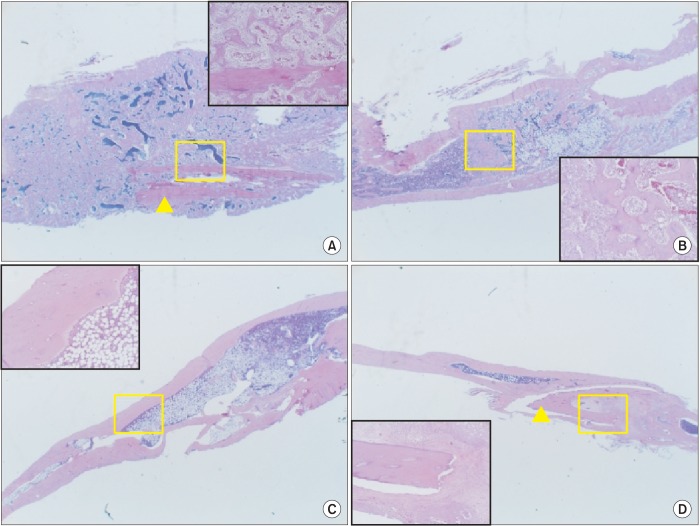
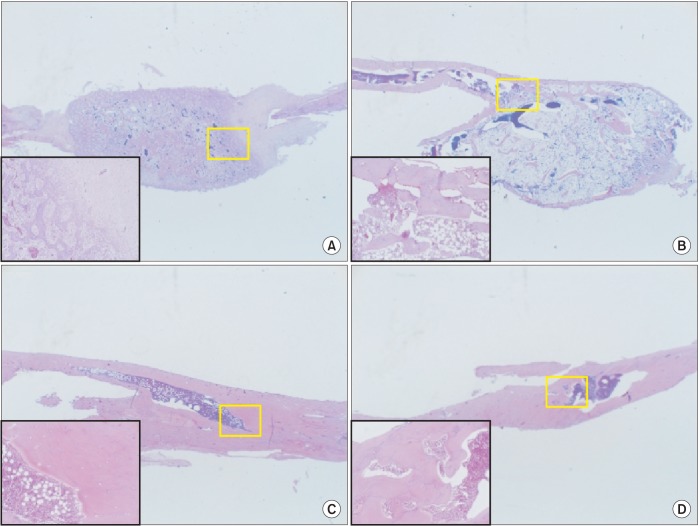
The purpose of this study was to introduce our three experiments on bone morphogenetic protein (BMP) and its carriers performed using the critical sized segmental defect (CSD) model in rat fibula and to investigate development of animal models and carriers for more effective bone regeneration.
MATERIALS AND METHODS
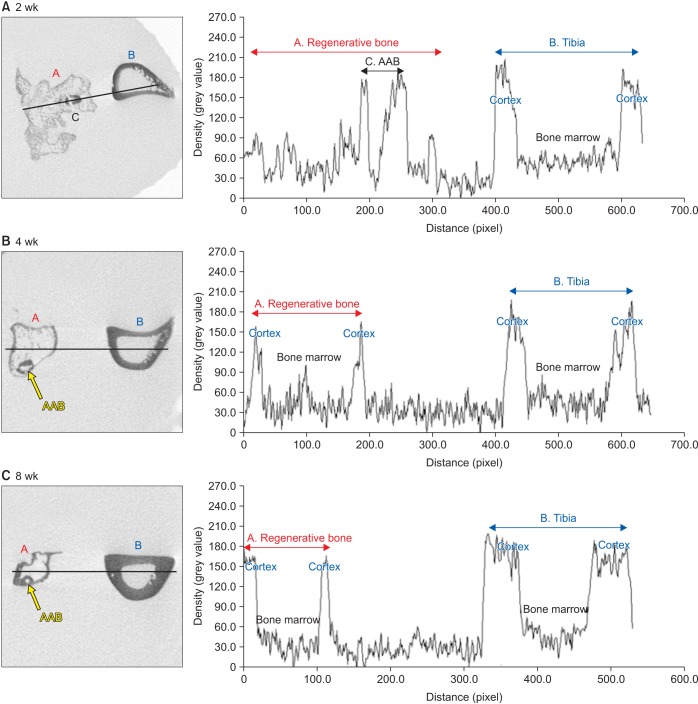
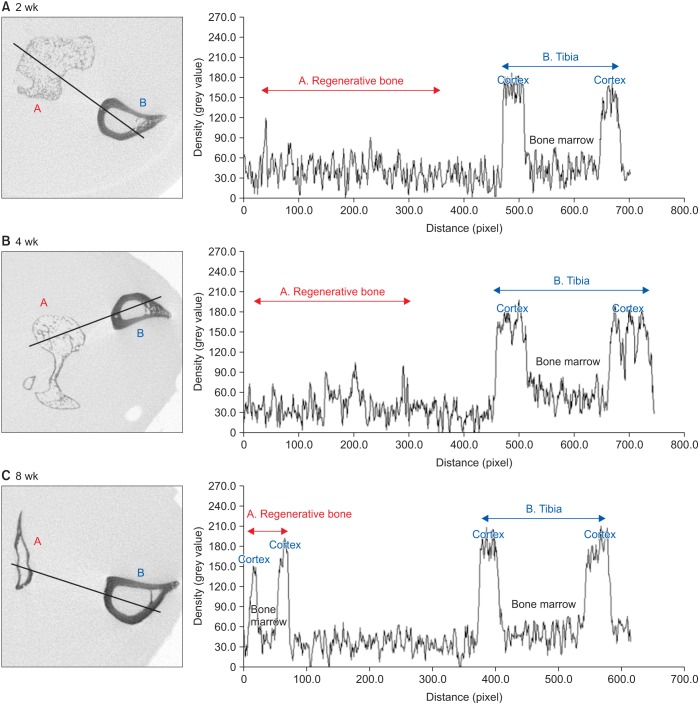
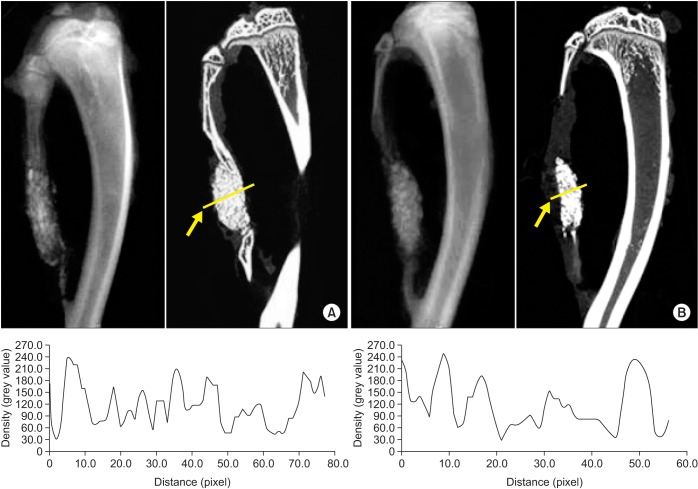
For the experiments, 14, 16, and 24 rats with CSDs on both fibulae were used in Experiments 1, 2, and 3, respectively. BMP-2 with absorbable collagen sponge (ACS) (Experiments 1 and 2), autoclaved autogenous bone (AAB) and fibrin glue (FG) (Experiment 3), and xenogenic bone (Experiment 2) were used in the experimental groups. Radiographic and histomorphological evaluations were performed during the follow-up period of each experiment.
RESULTS
Significant new bone formation was commonly observed in all experimental groups using BMP-2 compared to control and xenograft (porcine bone) groups. Although there was some difference based on BMP carrier, regenerated bone volume was typically reduced by remodeling after initially forming excessive bone.
CONCLUSION
BMP-2 demonstrates excellent ability for bone regeneration because of its osteoinductivity, but efficacy can be significantly different depending on its delivery system. ACS and FG showed relatively good bone regeneration capacity, satisfying the essential conditions of localization and release-control when used as BMP carriers. AAB could not provide release-control as a BMP carrier, but its space-maintenance role was remarkable. Carriers and scaffolds that can provide sufficient support to the BMP/carrier complex are necessary for large bone defects, and AAB is thought to be able to act as an effective scaffold. The CSD model of rat fibula is simple and useful for initial estimate of bone regeneration by agents including BMPs.
Keyword
MeSH Terms
Figure
Reference
-
1. Boyne PJ. Osseous reconstruction of the maxilla and the mandible: surgical techniques using titanium mesh and bone mineral. Implant Dent. 1998; 7:378–379.2. Hausamen JE, Neukam FW. Transplantation of bones. Eur Arch Otorhinolaryngol Suppl. 1992; 1:163–177. PMID: 1521069.3. Hopp SG, Dahners LE, Gilbert JA. A study of the mechanical strength of long bone defects treated with various bone autograft substitutes: an experimental investigation in the rabbit. J Orthop Res. 1989; 7:579–584. PMID: 2544712.
Article4. Dickinson BP, Ashley RK, Wasson KL, O'Hara C, Gabbay J, Heller JB, et al. Reduced morbidity and improved healing with bone morphogenic protein-2 in older patients with alveolar cleft defects. Plast Reconstr Surg. 2008; 121:209–217. PMID: 18176223.
Article5. Fallucco MA, Carstens MH. Primary reconstruction of alveolar clefts using recombinant human bone morphogenic protein-2: clinical and radiographic outcomes. J Craniofac Surg. 2009; 20(Suppl 2):1759–1764. PMID: 19816345.6. Herford AS. rhBMP-2 as an option for reconstructing mandibular continuity defects. J Oral Maxillofac Surg. 2009; 67:2679–2684. PMID: 19925991.
Article7. Herford AS, Boyne PJ. Reconstruction of mandibular continuity defects with bone morphogenetic protein-2 (rhBMP-2). J Oral Maxillofac Surg. 2008; 66:616–624. PMID: 18355584.
Article8. Hollinger JO, Kleinschmidt JC. The critical size defect as an experimental model to test bone repair materials. J Craniofac Surg. 1990; 1:60–68. PMID: 1965154.
Article9. Mapara M, Thomas BS, Bhat KM. Rabbit as an animal model for experimental research. Dent Res J (Isfahan). 2012; 9:111–118. PMID: 22363373.10. Choi EJ, Kang SH, Kwon HJ, Cho SW, Kim HJ. Bone healing properties of autoclaved autogenous bone grafts incorporating recombinant human bone morphogenetic protein-2 and comparison of two delivery systems in a segmental rabbit radius defect. Maxillofac Plast Reconstr Surg. 2014; 36:94–102. PMID: 27489818.
Article11. Boyne PJ, Marx RE, Nevins M, Triplett G, Lazaro E, Lilly LC, et al. A feasibility study evaluating rhBMP-2/absorbable collagen sponge for maxillary sinus floor augmentation. Int J Periodontics Restorative Dent. 1997; 17:11–25. PMID: 10332250.12. Lee JH, Kim CS, Choi KH, Jung UW, Yun JH, Choi SH, et al. The induction of bone formation in rat calvarial defects and subcutaneous tissues by recombinant human BMP-2, produced in Escherichia coli. Biomaterials. 2010; 31:3512–3519. PMID: 20149447.
Article13. McKay WF, Peckham SM, Badura JM. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft). Int Orthop. 2007; 31:729–734. PMID: 17639384.
Article14. Kübler NR, Reuther JF, Faller G, Kirchner T, Ruppert R, Sebald W. Inductive properties of recombinant human BMP-2 produced in a bacterial expression system. Int J Oral Maxillofac Surg. 1998; 27:305–309. PMID: 9698181.
Article15. Haidar ZS, Hamdy RC, Tabrizian M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part A: current challenges in BMP delivery. Biotechnol Lett. 2009; 31:1817–1824. PMID: 19690804.
Article16. Haidar ZS, Hamdy RC, Tabrizian M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part B: delivery systems for BMPs in orthopaedic and craniofacial tissue engineering. Biotechnol Lett. 2009; 31:1825–1835. PMID: 19690811.
Article17. Issa JP, Bentley MV, Iyomasa MM, Sebald W, De Albuquerque RF. Sustained release carriers used to delivery bone morphogenetic proteins in the bone healing process. Anat Histol Embryol. 2008; 37:181–187. PMID: 18070240.
Article18. Jung RE, Weber FE, Thoma DS, Ehrbar M, Cochran DL, Hämmerle CH. Bone morphogenetic protein-2 enhances bone formation when delivered by a synthetic matrix containing hydroxyapatite/tricalciumphosphate. Clin Oral Implants Res. 2008; 19:188–195. PMID: 18067602.
Article19. Wikesjö UM, Qahash M, Huang YH, Xiropaidis A, Polimeni G, Susin C. Bone morphogenetic proteins for periodontal and alveolar indications; biological observations--clinical implications. Orthod Craniofac Res. 2009; 12:263–270. PMID: 19627529.20. Han DK, Kim CS, Jung UW, Chai JK, Choi SH, Kim CK, et al. Effect of a fibrin-fibronectin sealing system as a carrier for recombinant human bone morphogenetic protein-4 on bone formation in rat calvarial defects. J Periodontol. 2005; 76:2216–2222. PMID: 16332232.
Article21. Hong SJ, Kim CS, Han DK, Cho IH, Jung UW, Choi SH, et al. The effect of a fibrin-fibronectin/beta-tricalcium phosphate/recombinant human bone morphogenetic protein-2 system on bone formation in rat calvarial defects. Biomaterials. 2006; 27:3810–3816. PMID: 16574220.22. Patel VV, Zhao L, Wong P, Kanim L, Bae HW, Pradhan BB, et al. Controlling bone morphogenetic protein diffusion and bone morphogenetic protein-stimulated bone growth using fibrin glue. Spine (Phila Pa 1976). 2006; 31:1201–1206. PMID: 16688032.
Article23. Kim HJ, Kang SW, Lim HC, Han SB, Lee JS, Prasad L, et al. The role of transforming growth factor-beta and bone morphogenetic protein with fibrin glue in healing of bone-tendon junction injury. Connect Tissue Res. 2007; 48:309–315. PMID: 18075817.24. Patel VV, Zhao L, Wong P, Pradhan BB, Bae HW, Kanim L, et al. An in vitro and in vivo analysis of fibrin glue use to control bone morphogenetic protein diffusion and bone morphogenetic protein-stimulated bone growth. Spine J. 2006; 6:397–403. discussion 404. PMID: 16825045.
Article25. Lee JG, Lee EW. Healing process of the reimplanted autoclaved autogenous mandible in adult dogs. J Korean Assoc Oral Maxillofac Surg. 1993; 19:514–532.26. Böhm P, Springfeld R, Springer H. Re-implantation of autoclaved bone segments in musculoskeletal tumor surgery. Clinical experience in 9 patients followed for 1.1–8.4 years and review of the literature. Arch Orthop Trauma Surg. 1998; 118:57–65. PMID: 9833108.27. Ito T, Sakano S, Sato K, Sugiura H, Iwata H, Murata Y, et al. Sensitivity of osteoinductive activity of demineralized and defatted rat femur to temperature and duration of heating. Clin Orthop Relat Res. 1995; (316):267–275. PMID: 7634716.
Article28. Kao JT, Comstock C. Reimplantation of a contaminated and devitalized bone fragment after autoclaving in an open fracture. J Orthop Trauma. 1995; 9:336–340. PMID: 7562157.
Article29. Sugiura H, Yamamura S, Sato K, Katagiri H, Nishida Y, Nakashima H, et al. Remodelling and healing process of moderately heat-treated bone grafts after wide resection of bone and soft-tissue tumors. Arch Orthop Trauma Surg. 2003; 123:514–520. PMID: 12844230.
Article30. von Wilmowsky C, Schwarz S, Kerl JM, Srour S, Lell M, Felszeghy E, et al. Reconstruction of a mandibular defect with autogenous, autoclaved bone grafts and tissue engineering: an in vivo pilot study. J Biomed Mater Res A. 2010; 93:1510–1518. PMID: 20014295.
Article31. Izawa H, Hachiya Y, Kawai T, Muramatsu K, Narita Y, Ban N, et al. The effect of heat-treated human bone morphogenetic protein on clinical implantation. Clin Orthop Relat Res. 2001; (390):252–258.
Article32. Smith WS, Struhl S. Replantation of an autoclaved autogenous segment of bone for treatment of chondrosarcoma. Long-term follow up. J Bone Joint Surg Am. 1988; 70:70–75. PMID: 3275675.
Article33. Shimizu K, Masumi S, Yano H, Fukunaga T, Ikebe S, Shin S. Revascularization and new bone formation in heat-treated bone grafts. Arch Orthop Trauma Surg. 1999; 119:57–61. PMID: 10076946.
Article34. Shin S, Yano H, Fukunaga T, Ikebe S, Shimizu K, Kaku N, et al. Biomechanical properties of heat-treated bone grafts. Arch Orthop Trauma Surg. 2005; 125:1–5. PMID: 15558293.
Article35. Crespi R, Capparè P, Gherlone E. Comparison of magnesium-enriched hydroxyapatite and porcine bone in human extraction socket healing: a histologic and histomorphometric evaluation. Int J Oral Maxillofac Implants. 2011; 26:1057–1062. PMID: 22010090.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Analysis of Bone regenerative effect with carriers of bone morphogenetic protein in rat calvarial defects
- Combined effect of bisphosphonate and recombinant human bone morphogenetic protein 2 on bone healing of rat calvarial defects
- Effect of composite of bone morphogenetic protein and plaster of paris on healing of bone defect in the rat tibia
- Effects of rhBMP-2 with various carriers on bone regeneration in rat calvarial defect
- Effect of protein transduction domain fused-bone morphogenetic protein-2 on bone regeneration in rat calvarial defects