J Breast Cancer.
2017 Dec;20(4):361-367. 10.4048/jbc.2017.20.4.361.
Wnt7a Deficiency Could Predict Worse Disease-Free and Overall Survival in Estrogen Receptor-Positive Breast Cancer
- Affiliations
-
- 1Department of Pathology, Hanyang University College of Medicine, Seoul, Korea. sspaik@hanyang.ac.kr
- 2Department of Surgery, Hanyang University College of Medicine, Seoul, Korea.
- KMID: 2398206
- DOI: http://doi.org/10.4048/jbc.2017.20.4.361
Abstract
- PURPOSE
Wnt7a is a glycoprotein involved in embryonic development and the progression of different types of malignant tumors. This study aimed to detect the level of Wnt7a expression in breast cancer and explore its role in the disease progression and prognosis.
METHODS
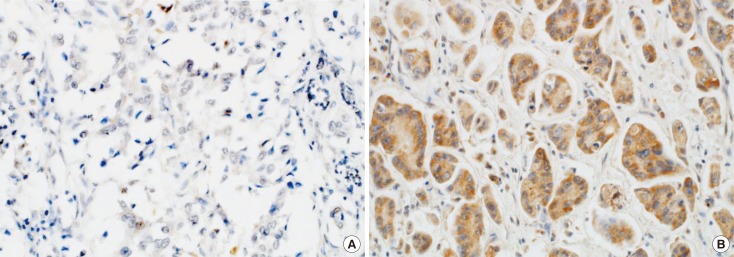
A total of 258 patients diagnosed with invasive ductal carcinoma of the breast were included in this study. Using tissue microarray and immunohistochemical staining, we evaluated the association between Wnt7a expression and clinicopathological parameters, and the prognostic value of Wnt7a.
RESULTS
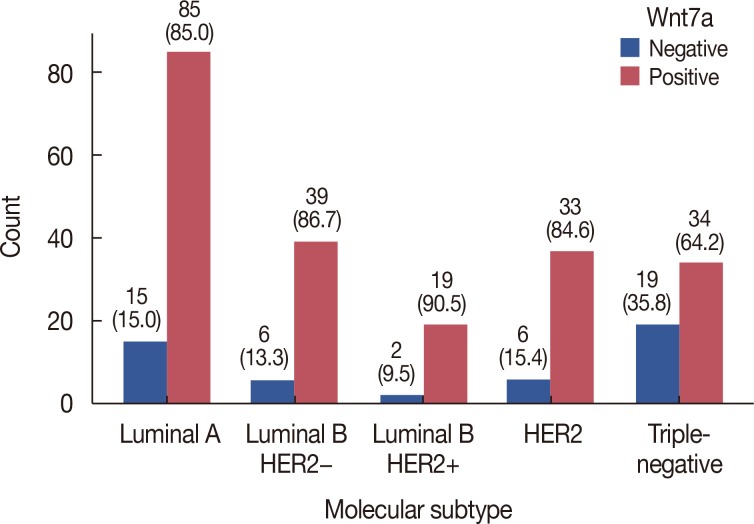
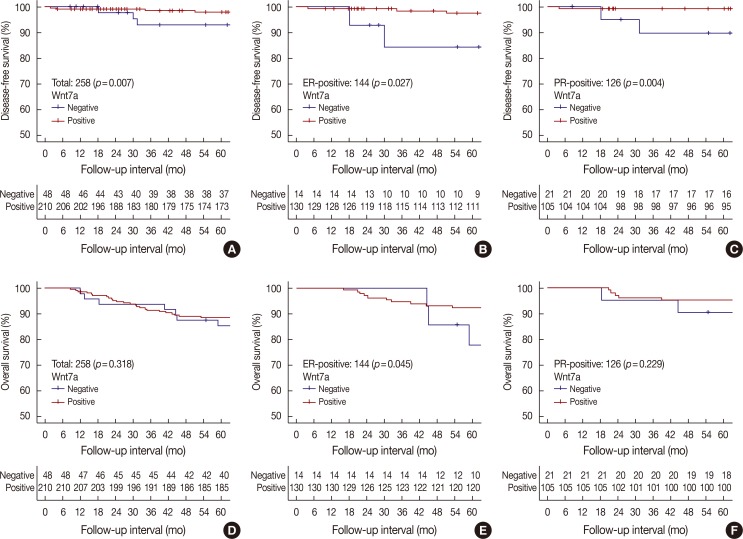
Wnt7a expression was significantly correlated with estrogen receptor (ER) expression (odds ratio, 3.95; 95% confidence interval [CI], 1.99-7.80; p < 0.001). On univariate and multivariate analyses, loss of Wnt7a expression was associated with poor disease-free survival (DFS) (multivariate hazard ratio [HR], 9.12; 95% CI, 1.80-46.09; p=0.008), but not with poor overall survival (OS). In the ER-positive group (n=114), loss of Wnt7a expression was an independent prognostic factor for shorter DFS (multivariate HR, 13.54; 95% CI, 1.11-165.73; p=0.042) and OS (multivariate HR, 4.76; 95% CI, 1.29-17.61; p=0.019) on univariate and multivariate analyses. However, in the ER-negative group, there was no significant difference in DFS and OS according to Wnt7a expression.
CONCLUSION
The loss of Wnt7a expression might be a meaningful factor in assessing DFS and OS, especially in ER-positive breast cancer.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Overexpression of
FRAT1 protein is closely related to triple-negative breast cancer
Sang Eun Nam, Young-Sin Ko, Kyoung Sik Park, TongYi Jin, Young-Bum Yoo, Jung-Hyun Yang, Wook-Youn Kim, Hye-Seung Han, So-Dug Lim, Seung Eun Lee, Wan-Seop Kim
Ann Surg Treat Res. 2022;103(2):63-71. doi: 10.4174/astr.2022.103.2.63.
Reference
-
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90. PMID: 21296855.
Article2. Dixon JM, Page DL, Anderson TJ, Lee D, Elton RA, Stewart HJ, et al. Long-term survivors after breast cancer. Br J Surg. 1985; 72:445–448. PMID: 4016513.
Article3. Jezierska A, Motyl T. Matrix metalloproteinase-2 involvement in breast cancer progression: a mini-review. Med Sci Monit. 2009; 15:RA32–RA40. PMID: 19182722.4. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, et al. Strategies for subtypes: dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011; 22:1736–1747. PMID: 21709140.5. Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004; 20:781–810. PMID: 15473860.
Article6. Kirikoshi H, Katoh M. Expression of WNT7A in human normal tissues and cancer, and regulation of WNT7A and WNT7B in human cancer. Int J Oncol. 2002; 21:895–900. PMID: 12239632.
Article7. Avgustinova A, Iravani M, Robertson D, Fearns A, Gao Q, Klingbeil P, et al. Tumour cell-derived Wnt7a recruits and activates fibroblasts to promote tumour aggressiveness. Nat Commun. 2016; 7:10305. PMID: 26777421.
Article8. Liu Y, Meng F, Xu Y, Yang S, Xiao M, Chen X, et al. Overexpression of Wnt7a is associated with tumor progression and unfavorable prognosis in endometrial cancer. Int J Gynecol Cancer. 2013; 23:304–311. PMID: 23321718.
Article9. Zhang XL, Peng CJ, Peng J, Jiang LY, Ning XM, Zheng JH. Prognostic role of Wnt7a expression in ovarian carcinoma patients. Neoplasma. 2010; 57:545–551. PMID: 20845993.
Article10. Ramos-Solano M, Meza-Canales ID, Torres-Reyes LA, Alvarez-Zavala M, Alvarado-Ruíz L, Rincon-Orozco B, et al. Expression of WNT genes in cervical cancer-derived cells: implication of WNT7A in cell proliferation and migration. Exp Cell Res. 2015; 335:39–50. PMID: 25978974.
Article11. Bikkavilli RK, Avasarala S, Van Scoyk M, Arcaroli J, Brzezinski C, Zhang W, et al. Wnt7a is a novel inducer of beta-catenin-independent tumor-suppressive cellular senescence in lung cancer. Oncogene. 2015; 34:5317–5328. PMID: 25728679.12. Ochoa-Hernández AB, Ramos-Solano M, Meza-Canales ID, García-Castro B, Rosales-Reynoso MA, Rosales-Aviña JA, et al. Peripheral T-lymphocytes express WNT7A and its restoration in leukemia-derived lymphoblasts inhibits cell proliferation. BMC Cancer. 2012; 12:60. PMID: 22313908.
Article13. Kondratov AG, Kvasha SM, Stoliar LA, Romanenko AM, Zgonnyk YM, Gordiyuk VV, et al. Alterations of the WNT7A gene in clear cell renal cell carcinomas. PLoS One. 2012; 7:e47012. PMID: 23056560.
Article14. Hirata T, Zheng Q, Chen Z, Kinoshita H, Okamoto J, Kratz J, et al. Wnt7A is a putative prognostic and chemosensitivity marker in human malignant pleural mesothelioma. Oncol Rep. 2015; 33:2052–2060. PMID: 25632963.
Article15. Peng C, Zhang X, Wang Y, Li L, Wang Q, Zheng J. Expression and prognostic significance of Wnt7a in human endometrial carcinoma. Obstet Gynecol Int. 2012; 2012:134962. PMID: 23304155.
Article16. Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987; 8:138–140. PMID: 3303008.17. Ohira T, Gemmill RM, Ferguson K, Kusy S, Roche J, Brambilla E, et al. WNT7a induces E-cadherin in lung cancer cells. Proc Natl Acad Sci U S A. 2003; 100:10429–10434. PMID: 12937339.
Article18. Yoshioka S, King ML, Ran S, Okuda H, MacLean JA 2nd, McAsey ME, et al. WNT7A regulates tumor growth and progression in ovarian cancer through the WNT/beta-catenin pathway. Mol Cancer Res. 2012; 10:469–482. PMID: 22232518.19. Hirabayashi Y, Itoh Y, Tabata H, Nakajima K, Akiyama T, Masuyama N, et al. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 2004; 131:2791–2801. PMID: 15142975.20. Lucas FR, Salinas PC. WNT-7a induces axonal remodeling and increases synapsin I levels in cerebellar neurons. Dev Biol. 1997; 192:31–44. PMID: 9405095.
Article21. Lyu J, Joo CK. Wnt-7a up-regulates matrix metalloproteinase-12 expression and promotes cell proliferation in corneal epithelial cells during wound healing. J Biol Chem. 2005; 280:21653–21660. PMID: 15802269.
Article22. Winn RA, Marek L, Han SY, Rodriguez K, Rodriguez N, Hammond M, et al. Restoration of Wnt-7a expression reverses non-small cell lung cancer cellular transformation through frizzled-9-mediated growth inhibition and promotion of cell differentiation. J Biol Chem. 2005; 280:19625–19634. PMID: 15705594.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment Outcomes of Weakly Positive Hormone Receptor Breast Cancer and Triple-Negative Breast Cancer
- Survival Benefit of Tamoxifen in Estrogen Receptor-Negative and Progesterone Receptor-Positive Low Grade Breast Cancer Patients
- Prognostic Value of Estrogen and Progesterone Receptor Expression in Low Proliferative Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer
- Clinical Differences in Triple-Positive Operable Breast Cancer Subtypes in Korean Patients: An Analysis of Korean Breast Cancer Registry Data
- Heterogeneity of the estrogen receptor in breast cancer tissues