Asia Pac Allergy.
2013 Oct;3(4):224-230. 10.5415/apallergy.2013.3.4.224.
Measuring and imaging small airways dysfunction in asthma
- Affiliations
-
- 1Department of Respiratory Medicine, Eastern Health and Monash University, Box Hill Hospital, Box Hill, VIC 3128, Australia. frank.thien@monash.edu
- KMID: 2397299
- DOI: http://doi.org/10.5415/apallergy.2013.3.4.224
Abstract
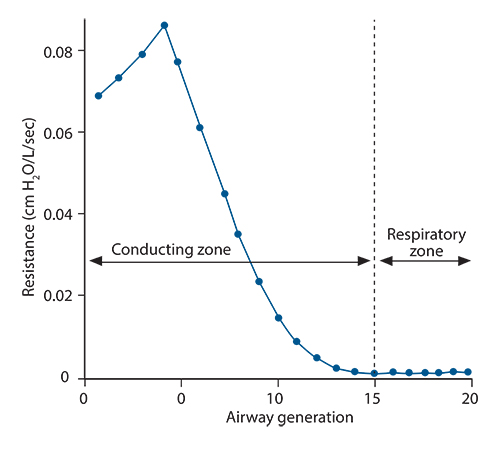
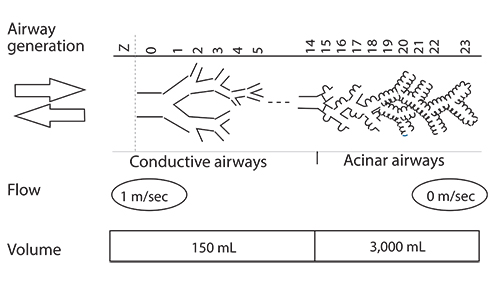
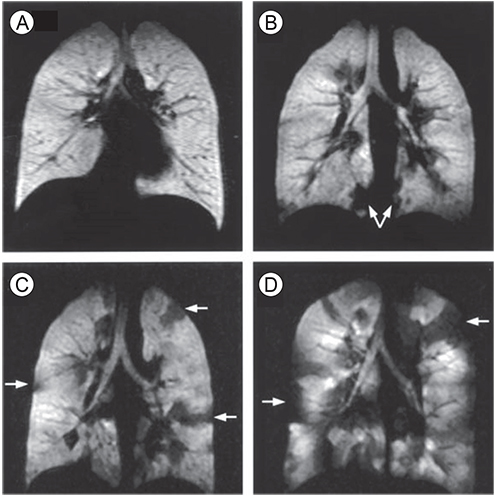
- Asthma is a chronic inflammatory disorder of the airways causing typical symptoms, and the diagnosis is supported by evidence of airflow obstruction which is variable, reversible or inducible. However, standard assessment of lung function with spirometry does not measure dysfunction in small airways which are < 2 mm in diameter towards the periphery of the lung. These airways make only a small contribution to airway resistance under normal circumstances. Nevertheless, there is mounting evidence that pathology and dysfunction in these small airways are implicated in the pathogenesis and natural history of asthma. Using forced oscillation and the multibreath nitrogen washout techniques, uneven ventilation (ventilation heterogeneity) due to small airways dysfunction has been shown to be an important marker of asthma disease activity, even in the absence of abnormalities in standard spirometric measurements. Recent advances in imaging research, particularly with hyperpolarised gas magnetic resonance imaging, have also given insights into the significance and dynamic nature of ventilation heterogeneity in asthma. The challenge is to integrate these new physiological and imaging insights to further our understanding of asthma and facilitate potential new treatments.
Keyword
MeSH Terms
Figure
Reference
-
1. Wenzel S. Severe asthma: from characteristics to phenotypes to endotypes. Clin Exp Allergy. 2012; 42:650–658.
Article2. Park D, Moore VC, Burge CB, Jaakkola MS, Robertson AS, Burge PS. Serial PEF measurement is superior to cross-shift change in diagnosing occupational asthma. Eur Respir J. 2009; 34:574–578.
Article3. Usmani OS, Barnes PJ. Assessing and treating small airways disease in asthma and chronic obstructive pulmonary disease. Ann Med. 2012; 44:146–156.
Article4. Baraldo S, Saetta M, Cosio MG. Pathophysiology of the small airways. Semin Respir Crit Care Med. 2003; 24:465–472.5. Macklem PT, Mead J. Resistance of central and peripheral airways measured by a retrograde catheter. J Appl Physiol. 1967; 22:395–401.
Article6. Despas PJ, Leroux M, Macklem PT. Site of airway obstruction in asthma as determined by measuring maximal expiratory flow breathing air and a helium-oxygen mixture. J Clin Invest. 1972; 51:3235–3243.
Article7. Wagner EM, Bleecker ER, Permutt S, Liu MC. Direct assessment of small airways reactivity in human subjects. Am J Respir Crit Care Med. 1998; 157:447–452.
Article8. Pride NB. Forced oscillation techniques for measuring mechanical properties of the respiratory system. Thorax. 1992; 47:317–320.
Article9. Oostveen E, MacLeod D, Lorino H, Farre R, Hantos Z, Desager K, Marchal F. ERS Task Force on Respiratory Impedance Measurements. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J. 2003; 22:1026–1041.
Article10. Skloot G, Goldman M, Fischler D, Goldman C, Schechter C, Levin S, Teirstein A. Respiratory symptoms and physiologic assessment of ironworkers at the World Trade Center disaster site. Chest. 2004; 125:1248–1255.
Article11. Cosio MG. Looking at the acinus with function tests: can you believe it? Am J Respir Crit Care Med. 2006; 174:847–848.12. Engel LA, Wood LD, Utz G, Macklem PT. Gas mixing during inspiration. J Appl Physiol. 1973; 35:18–24.
Article13. Verbanck S, Schuermans D, Van Muylem A, Paiva M, Noppen M, Vincken W. Ventilation distribution during histamine provocation. J Appl Physiol (1985). 1997; 83:1907–1916.
Article14. Thompson B, Probyn M, Neilsen K, Ng A, Matteo R, Harding R. Ventilation inhomogeneity in young lambs. Am J Resp Crit Care Med. 2007; 175:A818.15. Downie SR, Salome CM, Verbanck S, Thompson B, Berend N, King GG. Ventilation heterogeneity is a major determinant of airway hyperresponsiveness in asthma, independent of airway inflammation. Thorax. 2007; 62:684–689.
Article16. Venegas J. Linking ventilation heterogeneity and airway hyperresponsiveness in asthma. Thorax. 2007; 62:653–654.
Article17. Venegas JG, Winkler T, Musch G, Vidal Melo MF, Layfield D, Tgavalekos N, Fischman AJ, Callahan RJ, Bellani G, Harris RS. Self-organized patchiness in asthma as a prelude to catastrophic shifts. Nature. 2005; 434:777–782.
Article18. Thompson BR, Rees M, Ellis MJ, King GG, Douglass JA, Verbanck S. The severity of asthma is determined in the acinus. In : Proceedings of TSANZ 2008 Annual Scientific Meeting; 2008 Mar 28-April 4; Melbourne, Australia. TO56.19. Bourdin A, Paganin F, Prefaut C, Kieseler D, Godard P, Chanez P. Nitrogen washout slope in poorly controlled asthma. Allergy. 2006; 61:85–89.
Article20. Niimi A, Matsumoto H, Amitani R, Nakano Y, Mishima M, Minakuchi M, Nishimura K, Itoh H, Izumi T. Airway wall thickness in asthma assessed by computed tomography. Relation to clinical indices. Am J Respir Crit Care Med. 2000; 162:1518–1523.21. Kim WW, Lee CH, Goo JM, Park SJ, Kim JH, Park EA, Cho SH. Xenon-enhanced dual-energy CT of patients with asthma: dynamic ventilation changes after methacholine and salbutamol inhalation. AJR Am J Roentgenol. 2012; 199:975–981.
Article22. Park SJ, Lee CH, Goo JM, Kim JH, Park EA, Jung JW, Park HW, Cho SH. Quantitative analysis of dynamic airway changes after methacholine and salbutamol inhalation on xenon-enhanced chest CT. Eur Radiol. 2012; 22:2441–2450.
Article23. Jung JW, Kwon JW, Kim TW, Lee SH, Kim KM, Kang HR, Park HW, Lee CH, Goo JM, Min KU, Cho SH. New insight into the assessment of asthma using xenon ventilation computed tomography. Ann Allergy Asthma Immunol. 2013; 111:90–95.e2.
Article24. Phipatanakul W, Teague WG. Xenon ventilation computed tomography rules: new technology may open up further understanding in asthma. Ann Allergy Asthma Immunol. 2013; 111:81.
Article25. Albert MS, Cates GD, Driehuys B, Happer W, Saam B, Springer CS Jr, Wishnia A. Biological magnetic resonance imaging using laser-polarized 129Xe. Nature. 1994; 370:199–201.
Article26. MacFall JR, Charles HC, Black RD, Middleton H, Swartz JC, Saam B, Driehuys B, Erickson C, Happer W, Cates GD, Johnson GA, Ravin CE. Human lung air spaces: potential for MR imaging with hyperpolarized He-3. Radiology. 1996; 200:553–558.
Article27. Mugler JP 3rd, Driehuys B, Brookeman JR, Cates GD, Berr SS, Bryant RG, Daniel TM, de Lange EE, Downs JH 3rd, Erickson CJ, Happer W, Hinton DP, Kassel NF, Maier T, Phillips CD, Saam BT, Sauer KL, Wagshul ME. MR imaging and spectroscopy using hyperpolarized 129Xe gas: preliminary human results. Magn Reson Med. 1997; 37:809–815.28. Thien F, Friese M, Cowin G, Maillet D, Wang D, Galloway G, Brereton I, Robinson PJ, Heil W, Thompson B. Feasibility of functional magnetic resonance lung imaging in Australia with long distance transport of hyperpolarized helium from Germany. Respirology. 2008; 13:599–602.
Article29. Kauczor HU, Ebert M, Kreitner KF, Nilgens H, Surkau R, Heil W, Hofmann D, Otten EW, Thelen M. Imaging of the lungs using 3He MRI: preliminary clinical experience in 18 patients with and without lung disease. J Magn Reson Imaging. 1997; 7:538–543.
Article30. de Lange EE, Mugler JP 3rd, Brookeman JR, Knight-Scott J, Truwit JD, Teates CD, Daniel TM, Bogorad PL, Cates GD. Lung air spaces: MR imaging evaluation with hyperpolarized 3He gas. Radiology. 1999; 210:851–857.31. Samee S, Altes T, Powers P, de Lange EE, Knight-Scott J, Rakes G, Mugler JP 3rd, Ciambotti JM, Alford BA, Brookeman JR, Platts-Mills TA. Imaging the lungs in asthmatic patients by using hyperpolarized helium-3 magnetic resonance: assessment of response to methacholine and exercise challenge. J Allergy Clin Immunol. 2003; 111:1205–1211.
Article32. de Lange EE, Altes TA, Patrie JT, Battiston JJ, Juersivich AP, Mugler JP 3rd, Platts-Mills TA. Changes in regional airflow obstruction over time in the lungs of patients with asthma: evaluation with 3He MR imaging. Radiology. 2009; 250:567–575.33. de Lange EE, Altes TA, Patrie JT, Parmar J, Brookeman JR, Mugler JP 3rd, Platts-Mills TA. The variability of regional airflow obstruction within the lungs of patients with asthma: assessment with hyperpolarized helium-3 magnetic resonance imaging. J Allergy Clin Immunol. 2007; 119:1072–1078.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Small Airways Dysfunction in Asthma: Evaluation and Management to Improve Asthma Control
- Airway wall thickness and pulmonary functions in patients with bronchial asthma: Assessment with high resolution computed tomography (HRCT)
- Plasminogen Activator Inhibitor-1 in Asthma
- Does the Difference in Microbial Patterns in the Airways Induce Distinct Endotypes of Asthma?
- Lessons Learned From GWAS of Asthma