Asia Pac Allergy.
2014 Oct;4(4):187-196. 10.5415/apallergy.2014.4.4.187.
Role of inflammasome activation in development and exacerbation of asthma
- Affiliations
-
- 1Department of Interdisciplinary Program in Biomedical Science Major, Soonchunhyang University Graduate School, Bucheon 420-767, Korea.
- 2Genome Research Center for Allergy and Respiratory Disease, Division of Allergy and Respiratory Medicine, Soonchunhyang University Bucheon Hospital, Bucheon 420-767, Korea. mdcspark@daum.net
- KMID: 2397099
- DOI: http://doi.org/10.5415/apallergy.2014.4.4.187
Abstract
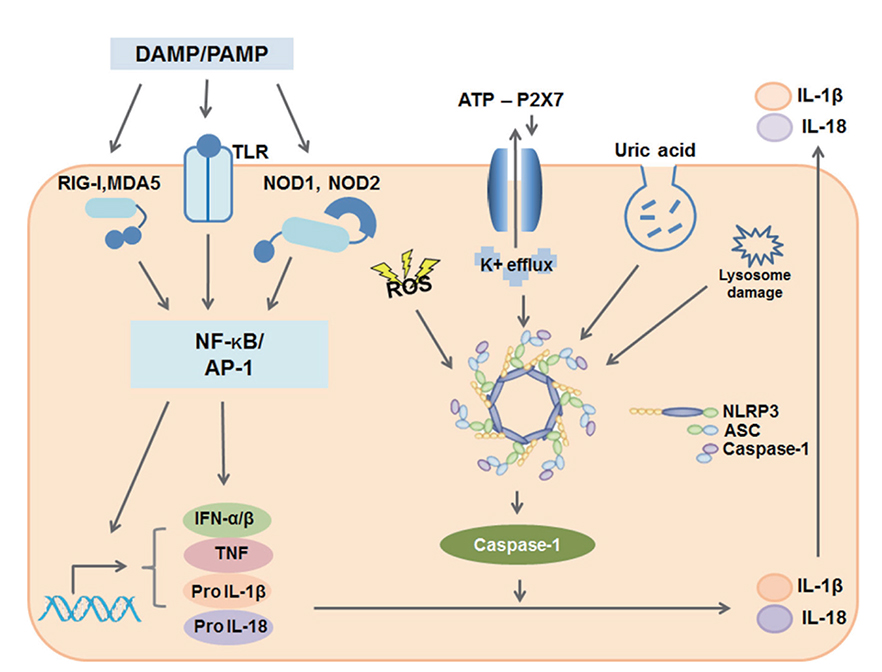
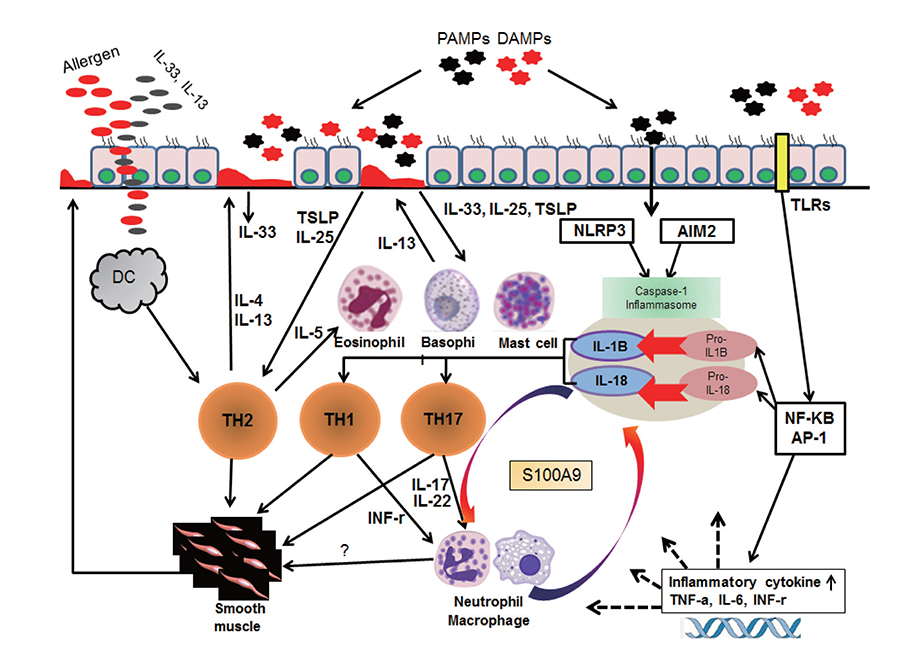
- Human airways contact with pathogen-associated molecular patterns and danger-associated molecular patterns present in many environments. Asthmatic's airways may be more susceptible to these patterns and lead to inflammasome activation; however, the participation of inflammasome in the development and exacerbation of asthma is not fully understood and remains controversial. Asthma is a heterogeneous group composed of different airway inflammation patterns with different underlying immune mechanisms. One mechanism is neutrophilic airway inflammation based on the axis of inflammasome activation, interleukin (IL) 1β/IL-18 production, T helper 17 activation, IL-8/IL-6 overproduction, and neutrophilic inflammation. The role of inflammasome activation has been highlighted in experimental asthma models and some evidence of inflammasome activation has been recently demonstrated in human neutrophilic asthmatic airways. In addition to caspase-1 activation, proteinase 3 and other protease from activated neutrophils directly cleave pro-IL-1β and pro-IL-18 to IL-1β and IL-18, which contribute to the phenotype of subsequent adaptive immune responses without inflammasome activation. Data suggests that neutrophilics in asthmatic airways may have an additional effect in initiating inflammasome activation and amplifying immune responses. Among the mediators from neutrophils, S100A9 seems to be one candidate mediator to explain the action of neutrophils in amplifying the airway inflammation in concert with inflammasome.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Effect of Ixeris dentata Nakai Extract on Nitric Oxide Production and Prostaglandin E2 Generation in LPS-stimulated RAW264.7 Cells
Yu Yeon Jung, Jin Tae Hong, Sang Bae Han, Young Hyun Park, Dong Ju Son
Immune Netw. 2015;15(6):325-330. doi: 10.4110/in.2015.15.6.325.In the memory of Professor Felicidad Cua-Lim
Yoon-Seok Chang
Asia Pac Allergy. 2014;4(4):185-186. doi: 10.5415/apallergy.2014.4.4.185.
Reference
-
1. Lecture on asthma-delivered at hotel Dieu, by Prof. Trousseau. Boston Med Surg J. 1859; 59:473–480.2. Holgate ST. The 1992 cournand lecture. Asthma: past, present and future. Eur Respir J. 1993; 6:1507–1520.3. Holgate ST. Mechanisms of asthma and implications for its prevention and treatment: a personal journey. Allergy Asthma Immunol Res. 2013; 5:343–347.
Article4. Ozato K, Shin DM, Chang TH, Morse HC 3rd. TRIM family proteins and their emerging roles in innate immunity. Nat Rev Immunol. 2008; 8:849–860.
Article5. Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, Rutschmann S, Du X, Hoebe K. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu Rev Immunol. 2006; 24:353–389.
Article6. Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002; 10:417–426.7. Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, Hayashi N, Higashino K, Okamura H, Nakanishi K, Kurimoto M, Tanimoto T, Flavell RA, Sato V, Harding MW, Livingston DJ, Su MS. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science. 1997; 275:206–209.8. Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci U S A. 2009; 106:9021–9026.
Article9. Zhang Y, Xu CB, Cardell LO. Long-term exposure to IL-1beta enhances Toll-IL-1 receptor-mediated inflammatory signaling in murine airway hyperresponsiveness. Eur Cytokine Netw. 2009; 20:148–156.10. Mahajan B, Vijayan VK, Agarwal MK, Bansal SK. Serum interleukin-1beta as a marker for differentiation of asthma and chronic obstructive pulmonary disease. Biomarkers. 2008; 13:713–727.11. Broide DH, Lotz M, Cuomo AJ, Coburn DA, Federman EC, Wasserman SI. Cytokines in symptomatic asthma airways. J Allergy Clin Immunol. 1992; 89:958–967.
Article12. Sousa AR, Trigg CJ, Lane SJ, Hawksworth R, Nakhosteen JA, Poston RN, Lee TH. Effect of inhaled glucocorticoids on IL-1 beta and IL-1 receptor antagonist (IL-1 ra) expression in asthmatic bronchial epithelium. Thorax. 1997; 52:407–410.
Article13. Ando M, Shima M. Serum interleukins 12 and 18 and immunoglobulin E concentrations and allergic symptoms in Japanese schoolchildren. J Investig Allergol Clin Immunol. 2007; 17:14–19.14. Patil SP, Wisnivesky JP, Busse PJ, Halm EA, Li XM. Detection of immunological biomarkers correlated with asthma control and quality of life measurements in sera from chronic asthmatic patients. Ann Allergy Asthma Immunol. 2011; 106:205–213.
Article15. Tanaka H, Miyazaki N, Oashi K, Teramoto S, Shiratori M, Hashimoto M, Ohmichi M, Abe S. IL-18 might reflect disease activity in mild and moderate asthma exacerbation. J Allergy Clin Immunol. 2001; 107:331–336.
Article16. Simpson JL, Phipps S, Baines KJ, Oreo KM, Gunawardhana L, Gibson PG. Elevated expression of the NLRP3 inflammasome in neutrophilic asthma. Eur Respir J. 2014; 43:1067–1076.
Article17. Schroder NW. The role of innate immunity in the pathogenesis of asthma. Curr Opin Allergy Clin Immunol. 2009; 9:38–43.18. Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009; 30:556–565.
Article19. Bauer RN, Brighton LE, Mueller L, Xiang Z, Rager JE, Fry RC, Peden DB, Jaspers I. Influenza enhances caspase-1 in bronchial epithelial cells from asthmatic volunteers and is associated with pathogenesis. J Allergy Clin Immunol. 2012; 130:958–967.e14.
Article20. Stout-Delgado HW, Vaughan SE, Shirali AC, Jaramillo RJ, Harrod KS. Impaired NLRP3 inflammasome function in elderly mice during influenza infection is rescued by treatment with nigericin. J Immunol. 2012; 188:2815–2824.
Article21. Triantafilou K, Kar S, van Kuppeveld FJ, Triantafilou M. Rhinovirus-induced calcium flux triggers NLRP3 and NLRC5 activation in bronchial cells. Am J Respir Cell Mol Biol. 2013; 49:923–934.
Article22. He X, Mekasha S, Mavrogiorgos N, Fitzgerald KA, Lien E, Ingalls RR. Inflammation and fibrosis during Chlamydia pneumoniae infection is regulated by IL-1 and the NLRP3/ASC inflammasome. J Immunol. 2010; 184:5743–5754.23. Dickson RP, Erb-Downward JR, Huffnagle GB. The role of the bacterial microbiome in lung disease. Expert Rev Respir Med. 2013; 7:245–257.
Article24. Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, Davies J, Ervine A, Poulter L, Pachter L, Moffatt MF, Cookson WO. Disordered microbial communities in asthmatic airways. PLoS One. 2010; 5:e8578.
Article25. Kool M, Petrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, Bergen IM, Castillo R, Lambrecht BN, Tschopp J. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008; 181:3755–3759.
Article26. Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008; 453:1122–1126.
Article27. Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Di Virgilio F, Virchow JC Jr, Lambrecht BN. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med. 2007; 13:913–919.
Article28. Hirota JA, Hirota SA, Warner SM, Stefanowicz D, Shaheen F, Beck PL, Macdonald JA, Hackett TL, Sin DD, Van Eeden S, Knight DA. The airway epithelium nucleotide-binding domain and leucine-rich repeat protein 3 inflammasome is activated by urban particulate matter. J Allergy Clin Immunol. 2012; 129:1116–1125.e6.
Article29. Allen IC, Jania CM, Wilson JE, Tekeppe EM, Hua X, Brickey WJ, Kwan M, Koller BH, Tilley SL, Ting JP. Analysis of NLRP3 in the development of allergic airway disease in mice. J Immunol. 2012; 188:2884–2893.
Article30. Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009; 27:519–550.
Article31. Yilmaz O, Sater AA, Yao L, Koutouzis T, Pettengill M, Ojcius DM. ATP-dependent activation of an inflammasome in primary gingival epithelial cells infected by Porphyromonas gingivalis. Cell Microbiol. 2010; 12:188–198.32. Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009; 30:576–587.
Article33. Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009; 31:331–341.34. Robinson D, Shibuya K, Mui A, Zonin F, Murphy E, Sana T, Hartley SB, Menon S, Kastelein R, Bazan F, O'Garra A. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-gamma production and activates IRAK and NFkappaB. Immunity. 1997; 7:571–581.35. Hayashi N, Yoshimoto T, Izuhara K, Matsui K, Tanaka T, Nakanishi K. T helper 1 cells stimulated with ovalbumin and IL-18 induce airway hyperresponsiveness and lung fibrosis by IFN-gamma and IL-13 production. Proc Natl Acad Sci U S A. 2007; 104:14765–14770.36. Besnard AG, Togbe D, Couillin I, Tan Z, Zheng SG, Erard F, Le Bert M, Quesniaux V, Ryffel B. Inflammasome-IL-1-Th17 response in allergic lung inflammation. J Mol Cell Biol. 2012; 4:3–10.
Article37. Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006; 368:804–813.
Article38. Robbins GR, Wen H, Ting JP. Inflammasomes and metabolic disorders: old genes in modern diseases. Mol Cell. 2014; 54:297–308.
Article39. Lamblin C, Gosset P, Tillie-Leblond I, Saulnier F, Marquette CH, Wallaert B, Tonnel AB. Bronchial neutrophilia in patients with noninfectious status asthmaticus. Am J Respir Crit Care Med. 1998; 157:394–402.
Article40. Jang AS, Kwon HS, Cho YS, Bae YJ, Kim TB, Park JS, Park SW, Uh ST, Choi JS, Kim YH, Hwang HK, Moon HB, Park CS. Identification of subtypes of refractory asthma in Korean patients by cluster analysis. Lung. 2013; 191:87–93.
Article41. Choi JS, Jang AS, Park JS, Park SW, Paik SH, Park JS, Uh ST, Kim YH, Park CS. Role of neutrophils in persistent airway obstruction due to refractory asthma. Respirology. 2012; 17:322–329.
Article42. Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN, Wenzel SE, Peters SP, Meyers DA, Bleecker ER. National Heart, Lung, and Blood Institute's Severe Asthma Research Program. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol. 2014; 133:1557–1563.e5.
Article43. Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, Ceuppens JL. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006; 7:135.
Article44. Ramirez-Velazquez C, Castillo EC, Guido-Bayardo L, Ortiz-Navarrete V. IL-17-producing peripheral blood CD177+ neutrophils increase in allergic asthmatic subjects. Allergy Asthma Clin Immunol. 2013; 9:23.
Article45. Sugawara S, Uehara A, Nochi T, Yamaguchi T, Ueda H, Sugiyama A, Hanzawa K, Kumagai K, Okamura H, Takada H. Neutrophil proteinase 3-mediated induction of bioactive IL-18 secretion by human oral epithelial cells. J Immunol. 2001; 167:6568–6575.
Article46. Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009; 15:410–416.
Article47. Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, Thorne PS, Wills-Karp M, Gioannini TL, Weiss JP, Karp CL. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009; 457:585–588.
Article48. Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol. 2008; 8:193–204.
Article49. Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008; 9:310–318.
Article50. Kouzaki H, Iijima K, Kobayashi T, O'Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011; 186:4375–4387.
Article51. Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel 'alarmin'? PLoS One. 2008; 3:e3331.
Article52. Guo L, Wei G, Zhu J, Liao W, Leonard WJ, Zhao K, Paul W. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc Natl Acad Sci U S A. 2009; 106:13463–13468.
Article53. Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010; 463:540–544.
Article54. Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005; 23:479–490.
Article55. Talabot-Ayer D, Lamacchia C, Gabay C, Palmer G. Interleukin-33 is biologically active independently of caspase-1 cleavage. J Biol Chem. 2009; 284:19420–19426.
Article56. Bae S, Kang T, Hong J, Lee S, Choi J, Jhun H, Kwak A, Hong K, Kim E, Jo S, Kim S. Contradictory functions (activation/termination) of neutrophil proteinase 3 enzyme (PR3) in interleukin-33 biological activity. J Biol Chem. 2012; 287:8205–8213.
Article57. Marenholz I, Heizmann CW, Fritz G. S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature). Biochem Biophys Res Commun. 2004; 322:1111–1122.
Article58. Ryckman C, Vandal K, Rouleau P, Talbot M, Tessier PA. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J Immunol. 2003; 170:3233–3242.
Article59. Kerkhoff C, Klempt M, Kaever V, Sorg C. The two calcium-binding proteins, S100A8 and S100A9, are involved in the metabolism of arachidonic acid in human neutrophils. J Biol Chem. 1999; 274:32672–32679.
Article60. Kannan S. Inflammation: a novel mechanism for the transport of extracellular nucleotide-induced arachidonic acid by S100A8/A9 for transcellular metabolism. Cell Biol Int. 2003; 27:593–595.
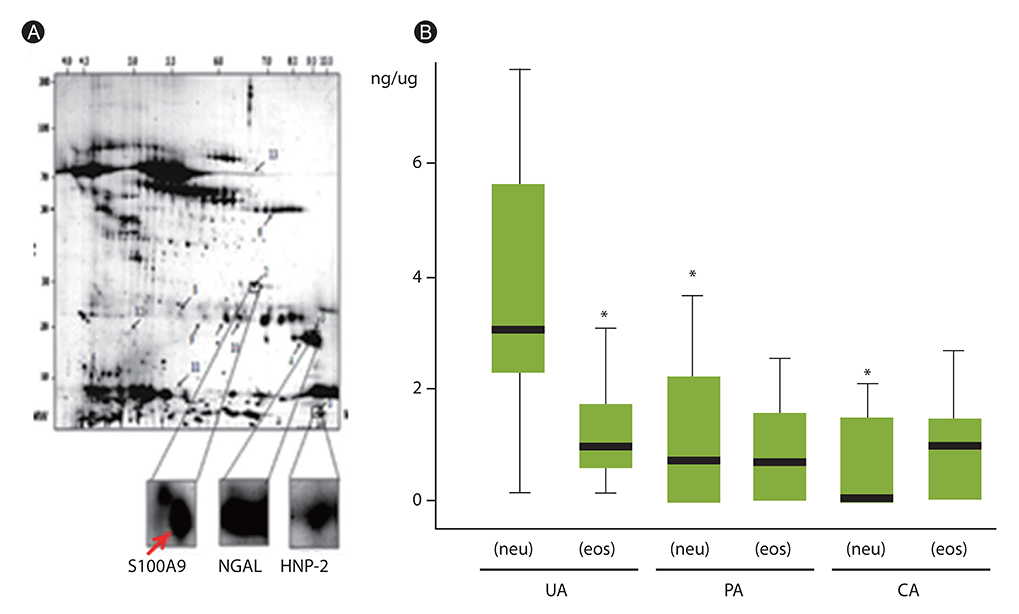
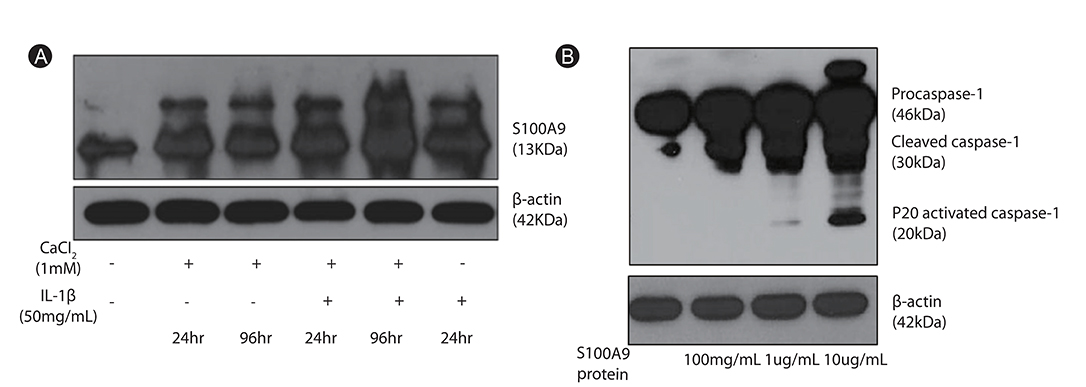
Article61. Kang JH, Hwang SM, Chung IY. S100A8, S100A9, and S100A12 activate airway epithelial cells to produce MUC5AC via ERK and NF-κB pathways. Immunology. 2014; 06. 30. [Epub]. http://dx.doi.org/10.1111/imm.12352.62. Lee TH, Jang AS, Park JS, Kim TH, Choi YS, Shin HR, Park SW, Uh ST, Choi JS, Kim YH, Kim Y, Kim S, Chung IY, Jeong SH, Park CS. Elevation of S100 calcium binding protein A9 in sputum of neutrophilic inflammation in severe uncontrolled asthma. Ann Allergy Asthma Immunol. 2013; 111:268–275.e1.
Article63. Xu X, Chen H, Zhu X, Ma Y, Liu Q, Xue Y, Chu H, Wu W, Wang J, Zou H. S100A9 promotes human lung fibroblast cells activation through receptor for advanced glycation end-product-mediated extracellular-regulated kinase 1/2, mitogen-activated protein-kinase and nuclear factor-κB-dependent pathways. Clin Exp Immunol. 2013; 173:523–535.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of air pollution on acute exacerbation of adult asthma in Seoul, Korea
- The Mechanism of the NLRP3 Inflammasome Activation and Pathogenic Implication in the Pathogenesis of Gout
- NLRP3 Inflammasome and Host Protection against Bacterial Infection
- The Role of NLR-related Protein 3 Inflammasome in Host Defense and Inflammatory Diseases
- Rhinovirus and childhood asthma: an update