Asia Pac Allergy.
2014 Jul;4(3):134-141. 10.5415/apallergy.2014.4.3.134.
Ocular toxocariasis: clinical features, diagnosis, treatment, and prevention
- Affiliations
-
- 1Department of Ophthalmology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam 463-707, Korea. sejoon1@snu.ac.kr
- 2Department of Ophthalmology, Armed Forces Capital Hospital, Seongnam 463-040, Korea.
- KMID: 2397090
- DOI: http://doi.org/10.5415/apallergy.2014.4.3.134
Abstract
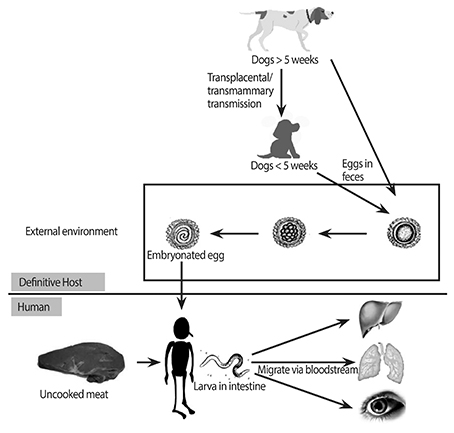
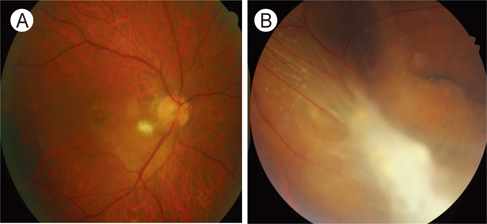
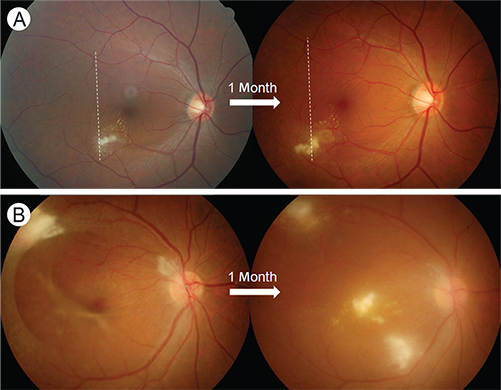
- Despite being one of the most common zoonotic infections worldwide, human toxocariasis has been one of the neglected tropical diseases. Although most human infections are asymptomatic, two main syndromes of human toxocariasis are classically recognized: systemic toxocariasis, which encompasses diseases in major organs; and ocular toxocariasis (OT), disease in the eye or optic nerve, caused by the migration of Toxocara larvae into the eye. OT is usually a unilateral disease, which typically presents as retinal granuloma, a yellowish or whitish inflammatory mass, in the posterior pole or peripheral retina. Granuloma itself or other comorbid conditions such as epiretinal membrane, macular edema, and retinal detachment can lead to permanent retinal damage and visual loss in eyes with OT. OT is diagnosed clinically by identification of clinical signs on ophthalmologic examination. Serological tests, such as enzyme-linked immunosorbent assay (ELISA) for detection of serum antibody against the Toxocara larvae, can confirm the diagnosis. In addition, serum immunoglobulin E and detection of ocular fluid antitoxocara antibody by ELISA may give additional aid to the diagnosis. Standard treatment of OT is corticosteroid in patients with active intraocular inflammation. Although the role of anthelmintic therapy is unclear, favorable outcome has been reported by combined corticosteroid and albendazole therapy in eyes with active inflammation. Prevention, by increasing public awareness and reducing the risk of infection, is also important. Recently, the association between ingestion of uncooked meat or liver and toxocariasis was reported, especially in adult patients. Future research on the potential source of infection, diagnosis, and treatment should be performed.
Keyword
MeSH Terms
-
Adult
Albendazole
Diagnosis*
Eating
Enzyme-Linked Immunosorbent Assay
Epiretinal Membrane
Granuloma
Humans
Immunoglobulin E
Immunoglobulins
Inflammation
Larva
Liver
Macular Edema
Meat
Optic Nerve
Retina
Retinal Detachment
Retinaldehyde
Serologic Tests
Toxocara
Toxocariasis*
Zoonoses
Albendazole
Immunoglobulin E
Immunoglobulins
Retinaldehyde
Figure
Cited by 2 articles
-
A Case of Spontaneous Closure of Macular Hole in Infectious Posterior Uveitis Involving the Fovea
Min Woo Kim, Dong Yoon Kim, Yoon Jeon Kim, Soo Geun Joe, Joo Yong Lee, June Gone Kim
J Korean Ophthalmol Soc. 2016;57(1):155-160. doi: 10.3341/jkos.2016.57.1.155.Atypical Fundus Manifestation of Ocular Toxocariasis
Joseph Kim, Hee Seung Chin
Korean J Ophthalmol. 2019;33(5):475-477. doi: 10.3341/kjo.2018.0121.
Reference
-
1. Rubinsky-Elefant G, Hirata CE, Yamamoto JH, Ferreira MU. Human toxocariasis: diagnosis, worldwide seroprevalences and clinical expression of the systemic and ocular forms. Ann Trop Med Parasitol. 2010; 104:3–23.
Article2. Despommier D. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin Microbiol Rev. 2003; 16:265–272.
Article3. Stensvold CR, Skov J, Moller LN, Jensen PM, Kapel CM, Petersen E, Nielsen HV. Seroprevalence of human toxocariasis in Denmark. Clin Vaccine Immunol. 2009; 16:1372–1373.
Article4. Fan CK, Hung CC, Du WY, Liao CW, Su KE. Seroepidemiology of Toxocara canis infection among mountain aboriginal schoolchildren living in contaminated districts in eastern Taiwan. Trop Med Int Health. 2004; 9:1312–1318.
Article5. Beaver PC, Snyder CH, Carrera GM, Dent JH, Lafferty JW. Chronic eosinophilia due to visceral larva migrans; report of three cases. Pediatrics. 1952; 9:7–19.6. Nichols RL. The etiology of visceral larva migrans. I. Diagnostic morphology of infective second-stage Toxocara larvae. J Parasitol. 1956; 42(4 Section 1):349–362.
Article7. Smith H, Holland C, Taylor M, Magnaval JF, Schantz P, Maizels R. How common is human toxocariasis? Towards standardizing our knowledge. Trends Parasitol. 2009; 25:182–188.
Article8. Biglan AW, Glickman LT, Lobes LA Jr. Serum and vitreous Toxocara antibody in nematode endophthalmitis. Am J Ophthalmol. 1979; 88:898–901.
Article9. Ahn SJ, Woo SJ, Jin Y, Chang YS, Kim TW, Ahn J, Heo JW, Yu HG, Chung H, Park KH, Hong ST. Clinical features and course of ocular toxocariasis in adults. PLoS Negl Trop Dis. 2014; 8:e2938.
Article10. Stewart JM, Cubillan LD, Cunningham ET Jr. Prevalence, clinical features, and causes of vision loss among patients with ocular toxocariasis. Retina. 2005; 25:1005–1013.
Article11. Maetz HM, Kleinstein RN, Federico D, Wayne J. Estimated prevalence of ocular toxoplasmosis and toxocariasis in Alabama. J Infect Dis. 1987; 156:414.
Article12. Good B, Holland CV, Taylor MR, Larragy J, Moriarty P, O'Regan M. Ocular toxocariasis in schoolchildren. Clin Infect Dis. 2004; 39:173–178.
Article13. Goto H, Mochizuki M, Yamaki K, Kotake S, Usui M, Ohno S. Epidemiological survey of intraocular inflammation in Japan. Jpn J Ophthalmol. 2007; 51:41–44.
Article14. Choi D, Lim JH, Choi DC, Paik SW, Kim SH, Huh S. Toxocariasis and ingestion of raw cow liver in patients with eosinophilia. Korean J Parasitol. 2008; 46:139–143.
Article15. Lim JH. Foodborne eosinophilia due to visceral larva migrans: a disease abandoned. J Korean Med Sci. 2012; 27:1–2.
Article16. Woodhall D, Starr MC, Montgomery SP, Jones JL, Lum F, Read RW, Moorthy RS. Ocular toxocariasis: epidemiologic, anatomic, and therapeutic variations based on a survey of ophthalmic subspecialists. Ophthalmology. 2012; 119:1211–1217.
Article17. Alabiad CR, Albini TA, Santos CI, Davis JL. Ocular Toxocariasis in a Seronegative Adult. Ophthalmic Surg Lasers Imaging. 2010; 04. 02. 1–3. [Epub]. http://dx.doi.org/10.3928/15428877-20100325-06.
Article18. Yokoi K, Goto H, Sakai J, Usui M. Clinical features of ocular toxocariasis in Japan. Ocul Immunol Inflamm. 2003; 11:269–275.
Article19. Mirdha BR, Khokar SK. Ocular toxocariasis in a North Indian population. J Trop Pediatr. 2002; 48:328–330.
Article20. Park SP, Park I, Park HY, Lee SU, Huh S, Magnaval JF. Five cases of ocular toxocariasis confirmed by serology. Korean J Parasitol. 2000; 38:267–273.
Article21. Gillespie SH, Dinning WJ, Voller A, Crowcroft NS. The spectrum of ocular toxocariasis. Eye (Lond). 1993; 7(Pt 3):415–418.
Article22. Yoshida M, Shirao Y, Asai H, Nagase H, Nakamura H, Okazawa T, Kondo K, Takayanagi TH, Fujita K, Akao N. A retrospective study of ocular toxocariasis in Japan: correlation with antibody prevalence and ophthalmological findings of patients with uveitis. J Helminthol. 1999; 73:357–361.
Article23. Wan WL, Cano MR, Pince KJ, Green RL. Echographic characteristics of ocular toxocariasis. Ophthalmology. 1991; 98:28–32.
Article24. Wilkinson CP, Welch RB. Intraocular toxocara. Am J Ophthalmol. 1971; 71:921–930.
Article25. Ahn SJ, Woo SJ, Hyon JY, Park KH. Cataract formation associated with ocular toxocariasis. J Cataract Refract Surg. 2013; 39:830–835.
Article26. Sivaratnam D, Subrayan V, Ali NA. Transvitreal migration of a Toxocara larva resulting in a second chorioretinal granuloma. Jpn J Ophthalmol. 2008; 52:416–417.
Article27. Suzuki T, Joko T, Akao N, Ohashi Y. Following the migration of a Toxocara larva in the retina by optical coherence tomography and fluorescein angiography. Jpn J Ophthalmol. 2005; 49:159–161.
Article28. Jacquier P, Gottstein B, Stingelin Y, Eckert J. Immunodiagnosis of toxocarosis in humans: evaluation of a new enzyme-linked immunosorbent assay kit. J Clin Microbiol. 1991; 29:1831–1835.
Article29. Schantz PM. Toxocara larva migrans now. Am J Trop Med Hyg. 1989; 41:3 Suppl. 21–34.
Article30. Hagler WS, Pollard ZF, Jarrett WH, Donnelly EH. Results of surgery for ocular Toxocara canis. Ophthalmology. 1981; 88:1081–1086.
Article31. Jin Y, Shen C, Huh S, Sohn WM, Choi MH, Hong ST. Serodiagnosis of toxocariasis by ELISA using crude antigen of Toxocara canis larvae. Korean J Parasitol. 2013; 51:433–439.
Article32. Loukas A, Mullin NP, Tetteh KK, Moens L, Maizels RM. A novel C-type lectin secreted by a tissue-dwelling parasitic nematode. Curr Biol. 1999; 9:825–828.
Article33. Sharkey JA, McKay PS. Ocular toxocariasis in a patient with repeatedly negative ELISA titre to Toxocara canis. Br J Ophthalmol. 1993; 77:253–254.
Article34. Elefant GR, Shimizu SH, Sanchez MC, Jacob CM, Ferreira AW. A serological follow-up of toxocariasis patients after chemotherapy based on the detection of IgG, IgA, and IgE antibodies by enzyme-linked immunosorbent assay. J Clin Lab Anal. 2006; 20:164–172.
Article35. Seo M, Yoon SC. A seroepidemiological survey of toxocariasis among eosinophilia patients in Chungcheongnam-do. Korean J Parasitol. 2012; 50:249–251.
Article36. Kim YH, Huh S, Chung YB. Seroprevalence of toxocariasis among healthy people with eosinophilia. Korean J Parasitol. 2008; 46:29–32.
Article37. Kwon NH, Oh MJ, Lee SP, Lee BJ, Choi DC. The prevalence and diagnostic value of toxocariasis in unknown eosinophilia. Ann Hematol. 2006; 85:233–238.
Article38. Arias Irigoyen J, Senent Sanchez CJ. Toxocariasis: a cause of hyper IgE and eosinophilia. J Investig Allergol Clin Immunol. 1995; 5:232–234.39. Shields JA. Ocular toxocariasis. A review. Surv Ophthalmol. 1984; 28:361–381.
Article40. Maguire AM, Green WR, Michels RG, Erozan YS. Recovery of intraocular Toxocara canis by pars plana vitrectomy. Ophthalmology. 1990; 97:675–680.
Article41. Barisani-Asenbauer T, Maca SM, Hauff W, Kaminski SL, Domanovits H, Theyer I, Auer H. Treatment of ocular toxocariasis with albendazole. J Ocul Pharmacol Ther. 2001; 17:287–294.
Article42. Bird AC, Smith JL, Curtin VT. Nematode optic neuritis. Am J Ophthalmol. 1970; 69:72–77.
Article43. Stürchler D, Schubarth P, Gualzata M, Gottstein B, Oettli A. Thiabendazole vs. albendazole in treatment of toxocariasis: a clinical trial. Ann Trop Med Parasitol. 1989; 83:473–478.44. Magnaval JF. Comparative efficacy of diethylcarbamazine and mebendazole for the treatment of human toxocariasis. Parasitology. 1995; 110(Pt 5):529–533.
Article45. Abdel-Hameed AA. Effect of thiabendazole on the migration of Toxocara canis larvae in the mouse. J Parasitol. 1984; 70:226–231.
Article46. Zygulska-Machowa H, Ziobrowski S. A case of ocular toxocariasis treated by xenon photocoagulation. Klin Oczna. 1987; 89:213–214.47. Lyall DA, Hutchison BM, Gaskell A, Varikkara M. Intravitreal Ranibizumab in the treatment of choroidal neovascularisation secondary to ocular toxocariasis in a 13-year-old boy. Eye (Lond). 2010; 24:1730–1731.
Article48. Giuliari GP, Ramirez G, Cortez RT. Surgical treatment of ocular toxocariasis: anatomic and functional results in 45 patients. Eur J Ophthalmol. 2011; 21:490–494.
Article49. Queiroz ML, Simonsen M, Paschoalotti MA, Chieffi PP. Frequency of soil contamination by Toxocara canis eggs in the south region of Sao Paulo municipality (SP, Brazil) in a 18 month period. Rev Inst Med Trop Sao Paulo. 2006; 48:317–319.50. Castillo D, Paredes C, Zanartu C, Castillo G, Mercado R, Munoz V, Schenone H. Environmental contamination with Toxocara sp. eggs in public squares and parks from Santiago, Chile, 1999. Bol Chil Parasitol. 2000; 55:86–91.51. Giacometti A, Cirioni O, Fortuna M, Osimani P, Antonicelli L, Del Prete MS, Riva A, D'Errico MM, Petrelli E, Scalise G. Environmental and serological evidence for the presence of toxocariasis in the urban area of Ancona, Italy. Eur J Epidemiol. 2000; 16:1023–1026.52. Akdemir C. Visceral larva migrans among children in Kütahya (Turkey) and an evaluation of playgrounds for T. canis eggs. Turk J Pediatr. 2010; 52:158–162.53. Borg OA, Woodruff AW. Prevalence of infective ova of Toxocara species in public places. Br Med J. 1973; 4:470–472.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ocular Inflammation Associated with Systemic Infection
- A Case of Ectopic Macula in Presumed Ocular Toxocariasis
- A case of presumed ocular toxocariasis in a 28-year old woman
- Atypical Form of Unilateral Optic Neuropathy Caused by Ocular Toxocariasis
- A Case Report of Intravitreal Dexamethasone Implant with Exudative Retinal Detachment for Ocular Toxocariasis Treatment