Korean Circ J.
2017 Nov;47(6):949-959. 10.4070/kcj.2016.0353.
Cardioprotective Effect of the SDF-1α/CXCR4 Axis in Ischemic Postconditioning in Isolated Rat Hearts
- Affiliations
-
- 1Cardiovascular Research Institute, Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Yangsan, Korea. ptca82@gmail.com
- 2Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3National Research Laboratory for Mitochondrial Signaling, Department of Physiology, Inje University College of Medicine, Busan, Korea.
- 4Department of Anesthesiology, University of North Carolina, Chapel Hill, NC, USA.
- 5Department of Chemistry, Pohang University of Science and Technology, Pohang, Korea.
- KMID: 2396488
- DOI: http://doi.org/10.4070/kcj.2016.0353
Abstract
- BACKGROUND AND OBJECTIVES
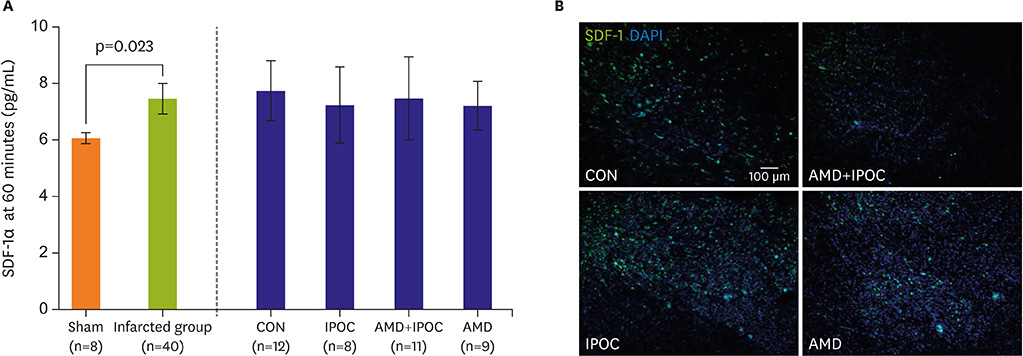
Information about the role of the stromal cell-derived factor-1α (SDF-1α)/chemokine receptor type 4 (CXCR4) axis in ischemic postconditioning (IPOC) is currently limited. We hypothesized that the SDF-1α/CXCR4 signaling pathway is directly involved in the cardioprotective effect of IPOC.
METHODS
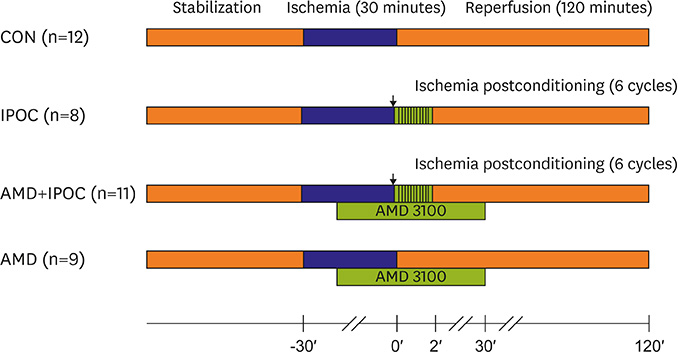
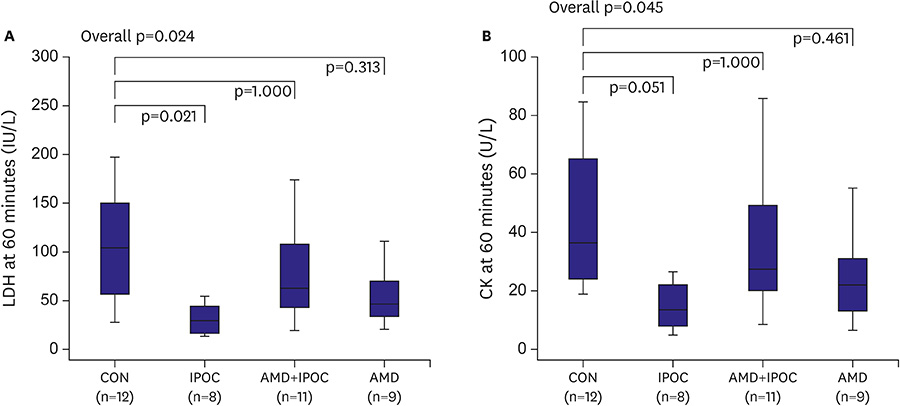
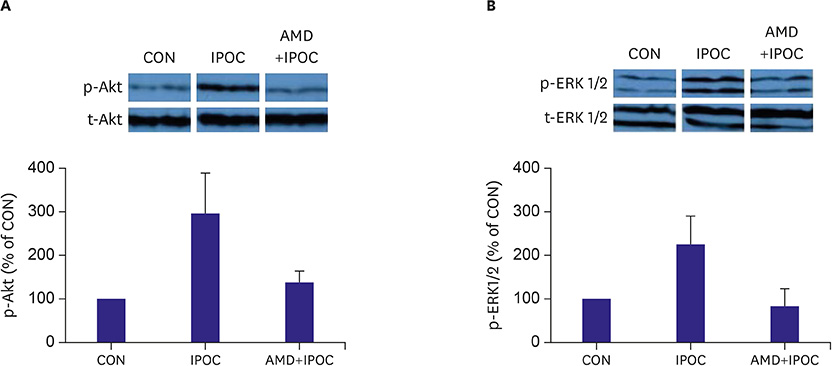
Isolated rat hearts were divided into four groups. The control group was subjected to 30-min of regional ischemia and 2-hour of reperfusion (n=12). The IPOC group was induced with 6 cycles of 10-second reperfusion and 10-second global ischemia (n=8) in each cycle. The CXCR4 antagonist, AMD3100, was applied before reperfusion in the IPOC group (AMD+IPOC group, n=11) and control group (AMD group, n=9). Hemodynamic changes with electrocardiography were monitored and infarct size was measured. The SDF-1α, lactate dehydrogenase (LDH) and creatine kinase (CK) concentrations in perfusate were measured. We also analyzed extracellular signal-regulated kinase 1/2 (ERK1/2) and Akt phosphorylation state expression.
RESULTS
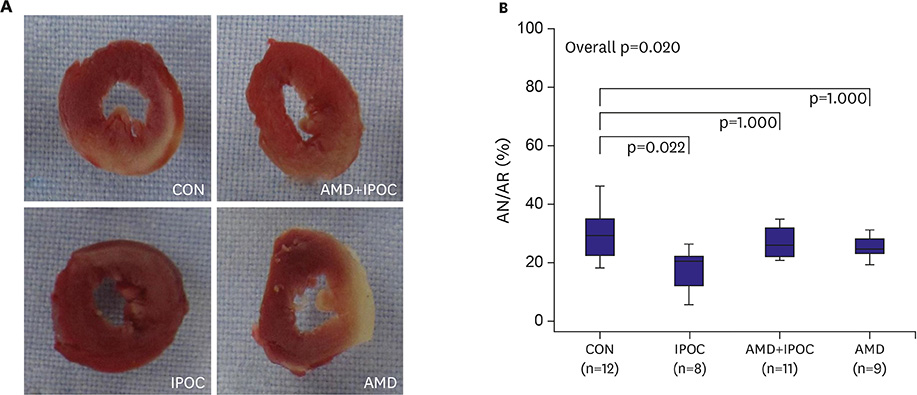
IPOC significantly reduced infarct size, but AMD3100 attenuated the infarct reducing effect of IPOC. IPOC significantly decreased LDH and CK, but these effects were reversed by AMD3100. ERK1/2 and Akt phosphorylation increased with IPOC and these effects were blocked by AMD3100.
CONCLUSION
Based on the results of this study, SDF-1α/CXCR4 signaling may be involved in IPOC cardioprotection and this signaling pathway couples to the ERK1/2 and Akt pathways.
Keyword
MeSH Terms
Figure
Reference
-
1. Kin H, Zhao ZQ, Sun HY, et al. Postconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovasc Res. 2004; 62:74–85.2. Tsang A, Hausenloy DJ, Mocanu MM, Yellon DM. Postconditioning: a form of “modified reperfusion” protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ Res. 2004; 95:230–232.3. Darling CE, Jiang R, Maynard M, Whittaker P, Vinten-Johansen J, Przyklenk K. Postconditioning via stuttering reperfusion limits myocardial infarct size in rabbit hearts: role of ERK1/2. Am J Physiol Heart Circ Physiol. 2005; 289:H1618–H1626.4. Hausenloy DJ, Tsang A, Yellon DM. The reperfusion injury salvage kinase pathway: a common target for both ischemic preconditioning and postconditioning. Trends Cardiovasc Med. 2005; 15:69–75.5. Jang Y, Xi J, Wang H, Mueller RA, Norfleet EA, Xu Z. Postconditioning prevents reperfusion injury by activating delta-opioid receptors. Anesthesiology. 2008; 108:243–250.6. Mykytenko J, Reeves JG, Kin H, et al. Persistent beneficial effect of postconditioning against infarct size: role of mitochondrial K(ATP) channels during reperfusion. Basic Res Cardiol. 2008; 103:472–484.7. Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004; 110:3300–3305.8. Askari AT, Unzek S, Popovic ZB, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003; 362:697–703.9. Hu X, Dai S, Wu WJ, et al. Stromal cell derived factor-1 alpha confers protection against myocardial ischemia/reperfusion injury: role of the cardiac stromal cell derived factor-1 alpha CXCR4 axis. Circulation. 2007; 116:654–663.10. Huang C, Gu H, Zhang W, Manukyan MC, Shou W, Wang M. SDF-1/CXCR4 mediates acute protection of cardiac function through myocardial STAT3 signaling following global ischemia/reperfusion injury. Am J Physiol Heart Circ Physiol. 2011; 301:H1496–H1505.11. Jang YH, Kim JH, Ban C, et al. Stromal cell derived factor-1 (SDF-1) targeting reperfusion reduces myocardial infarction in isolated rat hearts. Cardiovasc Ther. 2012; 30:264–272.12. van Vuuren D, Genis A, Genade S, Lochner A. Postconditioning the isolated working rat heart. Cardiovasc Drugs Ther. 2008; 22:391–397.13. Tachibana K, Hirota S, Iizasa H, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998; 393:591–594.14. Wei YJ, Tang Y, Li J, et al. Cloning and expression pattern of dog SDF-1 and the implications of altered expression of SDF-1 in ischemic myocardium. Cytokine. 2007; 40:52–59.15. Segret A, Rücker-Martin C, Pavoine C, et al. Structural localization and expression of CXCL12 and CXCR4 in rat heart and isolated cardiac myocytes. J Histochem Cytochem. 2007; 55:141–150.16. Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res. 2006; 70:240–253.17. Zhu M, Feng J, Lucchinetti E, et al. Ischemic postconditioning protects remodeled myocardium via the PI3K-PKB/Akt reperfusion injury salvage kinase pathway. Cardiovasc Res. 2006; 72:152–162.18. Yang XM, Proctor JB, Cui L, Krieg T, Downey JM, Cohen MV. Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. J Am Coll Cardiol. 2004; 44:1103–1110.19. Jujo K, Hamada H, Iwakura A, et al. CXCR4 blockade augments bone marrow progenitor cell recruitment to the neovasculature and reduces mortality after myocardial infarction. Proc Natl Acad Sci USA. 2010; 107:11008–11013.20. Chen Z, Li T, Zhang B. Morphine postconditioning protects against reperfusion injury in the isolated rat hearts. J Surg Res. 2008; 145:287–294.21. Methner C, Schmidt K, Cohen MV, Downey JM, Krieg T. Both A2a and A2b adenosine receptors at reperfusion are necessary to reduce infarct size in mouse hearts. Am J Physiol Heart Circ Physiol. 2010; 299:H1262–H1264.22. Peart JN, Gross GJ. Adenosine and opioid receptor-mediated cardioprotection in the rat: evidence for cross-talk between receptors. Am J Physiol Heart Circ Physiol. 2003; 285:H81–H89.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of postconditioning with N,N,N'N'-tetrakis-[2-pyridylmethyl]-ethylenediamine in isolated rat hearts
- Effect of Ischemic Preconditioning and Propofol on Myocardial Protection in the Globally Ischemic-reperfused Isolated Rat Heart
- Effect of Ischemic Preconditioning on Catecholamine Release from the Isolated, Ischemic Reperfused Hearts of Rats
- Morphine-induced postconditioning modulates mitochondrial permeability transition pore opening via delta-1 opioid receptors activation in isolated rat hearts
- The cardioprotective effect of microemulsion propofol against ischemia and reperfusion injury in isolated rat heart