Tuberc Respir Dis.
2017 Jan;80(1):77-82. 10.4046/trd.2017.80.1.77.
Mycobacterium tuberculosis ESAT6 and CPF10 Induce Adenosine Deaminase 2 mRNA Expression in Monocyte-Derived Macrophages
- Affiliations
-
- 1Department of Physiology, Cell and Matrix Research Institute, BK21 Plus KNU Biomedical Convergence Program, Tumor Heterogeneity and Network (THEN) Research Center, Kyungpook National University School of Medicine, Daegu, Korea.
- 2Department of Internal Medicine, Kyungpook National University School of Medicine, Daegu, Korea. kimch@knu.ac.kr jaelee@knu.ac.kr
- KMID: 2396344
- DOI: http://doi.org/10.4046/trd.2017.80.1.77
Abstract
- BACKGROUND
Delayed hypersensitivity plays a large role in the pathogenesis of tuberculous pleural effusion (TPE). Macrophages infected with live Mycobacterium tuberculosis (MTB) increase the levels of adenosine deaminase2 (ADA2) in the pleural fluid of TPE patients. However, it is as yet unclear whether ADA2 can be produced by macrophages when challenged with MTB antigens alone. This study therefore evaluated the levels of ADA2 mRNA expression, using monocyte-derived macrophages (MDMs) stimulated with MTB antigens.
METHODS
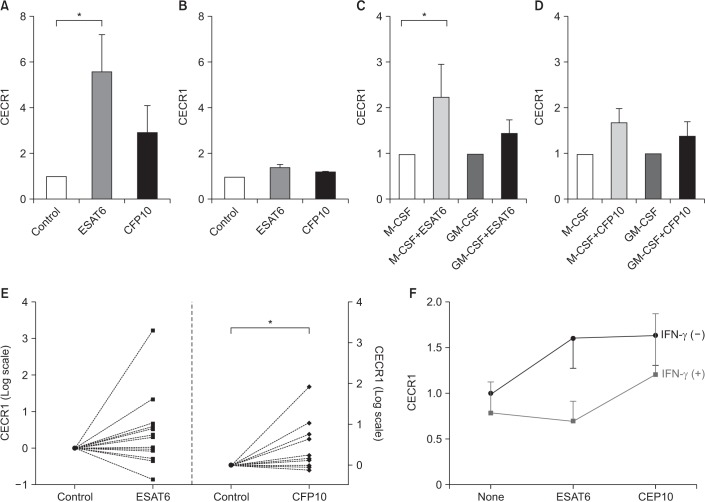
Purified monocytes from the peripheral blood mononuclear cells of healthy volunteers were differentiated into macrophages using granulocyte-macrophage colony-stimulating factor (GM-CSF) or macrophage colony-stimulating factor (M-CSF). The MDMs were stimulated with early secretory antigenic target protein 6 (ESAT6) and culture filtrate protein 10 (CFP10). The mRNA expression levels for the cat eye syndrome chromosome region, candidate 1 (CECR1) gene encoding ADA2 were then measured.
RESULTS
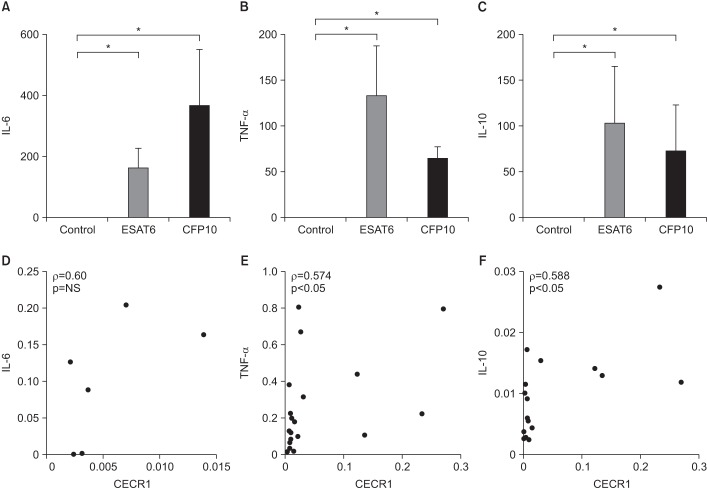
CECR1 mRNA expression levels were significantly higher in MDMs stimulated with ESAT6 and CFP10, than in the unstimulated MDMs. When stimulated with ESAT6, M-CSF-treated MDMs showed more pronounced CECR1 mRNA expression than GM-CSF-treated MDMs. Interferon-γ decreased the ESAT6- and CFP10-induced CECR1 mRNA expression in MDMs. CECR1 mRNA expression levels were positively correlated with mRNA expression of tumor necrosis factor α and interleukin 10, respectively.
CONCLUSION
ADA2 mRNA expression increased when MDMs were stimulated with MTB antigens alone. This partly indicates that pleural fluid ADA levels could increase in patients with culture-negative TPE. Our results may be helpful in improving the understanding of TPE pathogenesis.
Keyword
MeSH Terms
-
Adenosine Deaminase*
Adenosine*
Animals
Cats
Granulocyte-Macrophage Colony-Stimulating Factor
Healthy Volunteers
Humans
Hypersensitivity, Delayed
Interleukin-10
Macrophage Colony-Stimulating Factor
Macrophages*
Monocytes
Mycobacterium tuberculosis*
Mycobacterium*
Pleural Effusion
RNA, Messenger*
Tumor Necrosis Factor-alpha
Adenosine
Adenosine Deaminase
Granulocyte-Macrophage Colony-Stimulating Factor
Interleukin-10
Macrophage Colony-Stimulating Factor
RNA, Messenger
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Light RW. Update on tuberculous pleural effusion. Respirology. 2010; 15:451–458. PMID: 20345583.
Article2. Jeon D. Tuberculous pleurisy: an update. Tuberc Respir Dis. 2014; 76:153–159.
Article3. Allen JC, Apicella MA. Experimental pleural effusion as a manifestation of delayed hypersensitivity to tuberculin PPD. J Immunol. 1968; 101:481–487. PMID: 5692098.4. Leibowitz S, Kennedy L, Lessof MH. The tuberculin reaction in the pleural cavity and its suppression by antilymphocyte serum. Br J Exp Pathol. 1973; 54:152–162. PMID: 4700698.5. Yamamoto S, Dunn CJ, Willoughby DA. Studies on delayed hypersensitivity pleural exudates in guinea-pigs. II. The interrelationship of monocytic and lymphocytic cells with respect to migration activity. Immunology. 1976; 30:513–519. PMID: 131780.6. Lee JY. Diagnosis and treatment of extrapulmonary tuberculosis. Tuberc Respir Dis. 2015; 78:47–55.
Article7. Gakis C. Adenosine deaminase (ADA) isoenzymes ADA1 and ADA2: diagnostic and biological role. Eur Respir J. 1996; 9:632–633. PMID: 8726922.
Article8. Kashyap RS, Deshpande PS, Nayak AR, Purohit HJ, Taori GM, Daginawala HF. Adenosine deaminase activity in the supernatant of monocytes infected with Mycobacterium tuberculosis. Int J Integr Biol. 2007; 1:61–64.9. Kim CH, Lee J, Lee J, Cliff JM, Toulza F, Smith S, et al. Mycobacterial load affects adenosine deaminase 2 levels of tuberculous pleural effusion. J Infect. 2015; 71:488–491. PMID: 26049138.
Article10. Hasan Z, Jamil B, Ashraf M, Islam M, Dojki M, Irfan M, et al. Differential live Mycobacterium tuberculosis-, M. bovis BCG-, recombinant ESAT6-, and culture filtrate protein 10-induced immunity in tuberculosis. Clin Vaccine Immunol. 2009; 16:991–998. PMID: 19439524.11. Hasan Z, Schlax C, Kuhn L, Lefkovits I, Young D, Thole J, et al. Isolation and characterization of the mycobacterial phagosome: segregation from the endosomal/lysosomal pathway. Mol Microbiol. 1997; 24:545–553. PMID: 9179848.
Article12. Wongtim S, Silachamroon U, Ruxrungtham K, Udompanich V, Limthongkul S, Charoenlap P, et al. Interferon gamma for diagnosing tuberculous pleural effusions. Thorax. 1999; 54:921–924. PMID: 10491456.
Article13. Piras MA, Gakis C, Budroni M, Andreoni G. Adenosine deaminase activity in pleural effusions: an aid to differential diagnosis. Br Med J. 1978; 2:1751–1752.
Article14. Gakis C, Calia G, Naitana A, Pirino D, Serru G. Serum adenosine deaminase activity in HIV positive subjects: a hypothesis on the significance of ADA2. Panminerva Med. 1989; 31:107–113. PMID: 2689968.15. Gakis C, Cappio-Borlino A, Pulina G. Enzymes (isoenzyme system) as homeostatic mechanisms the isoenzyme (ADA2) of adenosine deaminase of human monocytes-macrophages as a regulator of the 2’deoxyadenosine. Biochem Mol Biol Int. 1998; 46:487–494. PMID: 9818088.
Article16. Tay TR, Tee A. Factors affecting pleural fluid adenosine deaminase level and the implication on the diagnosis of tuberculous pleural effusion: a retrospective cohort study. BMC Infect Dis. 2013; 13:546. PMID: 24238276.
Article17. Lee SJ, Kim HS, Lee SH, Lee TW, Lee HR, Cho YJ, et al. Factors influencing pleural adenosine deaminase level in patients with tuberculous pleurisy. Am J Med Sci. 2014; 348:362–365. PMID: 24762755.
Article18. Zavialov AV, Gracia E, Glaichenhaus N, Franco R, Lauvau G. Human adenosine deaminase 2 induces differentiation of monocytes into macrophages and stimulates proliferation of T helper cells and macrophages. J Leukoc Biol. 2010; 88:279–290. PMID: 20453107.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mycobacterium tuberculosis ESAT6 Drives the Activation and Maturation of Bone Marrow-Derived Dendritic Cells via TLR4-Mediated Signaling
- Adenosine deaminase activity in bronchoalveolar lavage fluid in patients with pulmonary tuberculosis
- Urine Adenosine Deaminase Activity in Confirmed Urinary Tract Tuberculosis
- A study on the diagnostic value of cerebrospinal fluid adenosine deaminase activity in children with tuberculous meningitis
- The Relationship between Age and Pleural Fluid Adenosine Deaminase Activity in Pleural Tuberculosis