Yonsei Med J.
2009 Apr;50(2):266-272.
Mutational Analysis of KRAS, BRAF, and TP53 Genes of Ovarian Serous Carcinomas in Korean Women
- Affiliations
-
- 1Department of Obstetrics and Gynecology, College of Medicine, University of Ulsan, Asan Medical Center, Seoul, Korea. ytkim@amc.seoul.kr
- 2Department of Pathology, College of Medicine, University of Ulsan, Asan Medical Center, Seoul, Korea.
Abstract
- PURPOSE
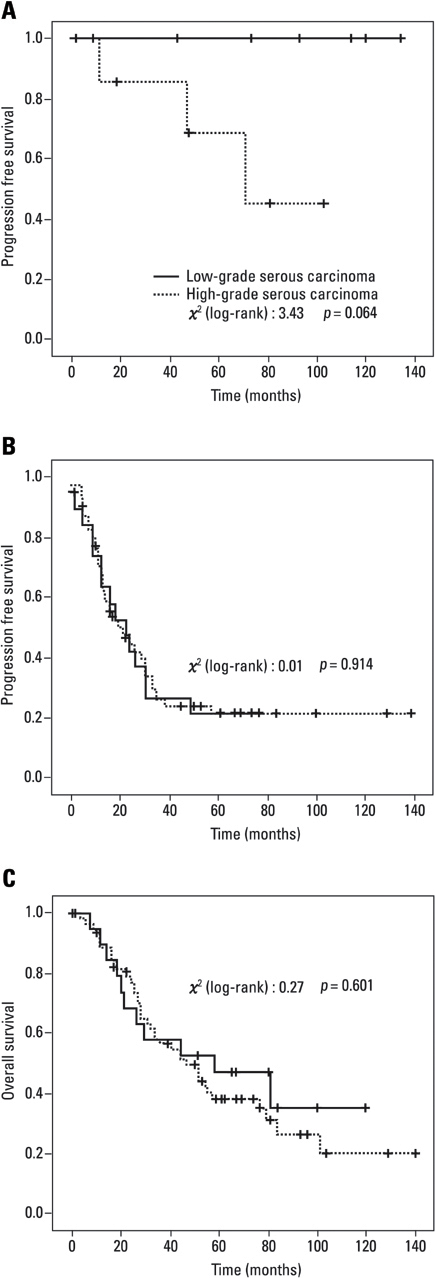
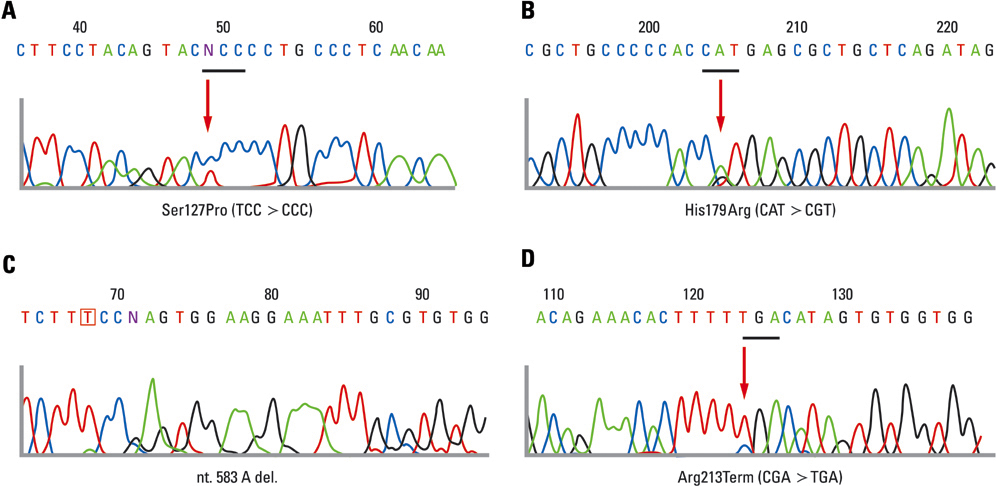
To assess the prevalence of KRAS, BRAF, and TP53 mutations in cases of low-grade and high-grade serous carcinomas and to evaluate the clinical outcomes of these morphologically distinct carcinomas. MATERIALS AND METHODS: Patients with primary invasive serous carcinomas were classified according to the universal grading system. Grade 2 serous tumors were excluded. A total of 100 patients were included for clinical evaluation. Thirty-seven patients, including 20 with low-grade and 17 with high-grade carcinomas, were selected for mutational analysis. RESULTS: The low-grade carcinoma group was characterized by young age and premenopausal period compared with the high-grade carcinoma group, but there were no statistically significant differences in stage, metastasis of lymph node and residual disease. There were no statistically significant differences in survival rates, however, the low-grade carcinoma group showed a trend for improved progression-free survival compared with the high-grade carcinoma group of early stage (p = 0.064). Mutations in KRAS and BRAF were found in 6 (30%) and 2 (10%) patients in the low-grade carcinoma group, respectively, however, they were not found in the high-grade carcinoma group. KRAS and BRAF mutations were mutually exclusive, and both mutations were observed in 40% (8/20). The frequency of TP53 mutations in low-grade and high-grade carcinoma groups were found in 20% (4/20) and 70.6% (12/17), respectively (p = 0.009). CONCLUSION: Low-grade serous carcinoma shows mutation pattern different from that with high-grade carcinoma. As there were no significant differences in stage distribution and survival, especially in advanced stage, we suggest that more studies are needed to segregate these patients into distinct disease entities.
Keyword
MeSH Terms
Figure
Reference
-
1. Willner J, Wurz K, Allison KH, Galic V, Garcia RL, Goff BA, et al. Alternate molecular genetic pathways in ovarian carcinomas of common histological types. Hum Pathol. 2007. 38:607–613.
Article2. Singer G, Kurman RJ, Chang HW, Cho SK, Shih IeM. Diverse tumorigenic pathways in ovarian serous carcinoma. Am J Pathol. 2002. 160:1223–1228.
Article3. Shih IeM, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004. 164:1511–1518.4. Singer G, Oldt R 3rd, Cohen Y, Wang BG, Sidransky D, Kurman RJ, et al. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst. 2003. 95:484–486.
Article5. Olson JM, Hallahan AR. p38 MAP kinase: a convergence point in cancer therapy. Trends Mol Med. 2004. 10:125–129.
Article6. Lang J, Boxer M, MacKie R. Absence of exon 15 BRAF germline mutations in familial melanoma. Hum Mutat. 2003. 21:327–330.7. Cohen Y, Xing M, Mambo E, Guo Z, Wu G, Trink B, et al. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst. 2003. 95:625–627.
Article8. Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003. 63:1454–1457.9. Naoki K, Chen TH, Richards WG, Sugarbaker DJ, Meyerson M. Missense mutations of the BRAF gene in human lung adenocarcinoma. Cancer Res. 2002. 62:7001–7003.10. Bell DA. Origins and molecular pathology of ovarian cancer. Mod Pathol. 2005. 18:Suppl 2. S19–S32.
Article11. Shih IeM, Kurman RJ. Molecular pathogenesis of ovarian borderline tumors: new insights and old challenges. Clin Cancer Res. 2005. 11:7273–7279.
Article12. Mammas IN, Zafiropoulos A, Spandidos DA. Involvement of the ras genes in female genital tract cancer. Int J Oncol. 2005. 26:1241–1255.13. Singer G, Stöhr R, Cope L, Dehari R, Hartmann A, Cao DF, et al. Patterns of p53 mutations separate ovarian serous borderline tumors and low-and high-grade carcinomas and provide support for a new model of ovarian carcinogenesis: a mutational analysis with immunohistochemical correlation. Am J Surg Pathol. 2005. 29:218–224.
Article14. Sieben NL, Macropoulos P, Roemen GM, Kolkman-Uljee SM, Jan Fleuren G, Houmadi R, et al. In ovarian neoplasms, BRAF, but not KRAS, mutations are restricted to low-grade serous tumours. J Pathol. 2004. 202:336–340.
Article15. Pohl G, Ho CL, Kurman RJ, Bristow R, Wang TL, Shih IeM. Inactivation of the mitogen-activated protein kinase pathway as a potential target-based therapy in ovarian serous tumors with KRAS or BRAF mutations. Cancer Res. 2005. 65:1994–2000.
Article16. Greenblatt MS, Bennett WP, Hollstein M, Harris CC. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994. 54:4855–4878.17. Kinzler KW, Vogelstein B. Cancer therapy meets p53. N Engl J Med. 1994. 331:49–50.
Article18. Russell SE, McCluggage WG. A multistep model for ovarian tumorigenesis: the value of mutation analysis in the KRAS and BRAF genes. J Pathol. 2004. 203:617–619.
Article19. Shimizu Y, Kamoi S, Amada S, Akiyama F, Silverberg SG. Toward the development of a universal grading system for ovarian epithelial carcinoma: testing of a proposed system in a series of 461 patients with uniform treatment and follow-up. Cancer. 1998. 82:893–901.
Article20. Shimizu Y, Kamoi S, Amada S, Hasumi K, Akiyama F, Silverberg SG. Toward the development of a universal grading system for ovarian epithelial carcinoma. I. Prognostic significance of histopathologic features--problems involved in the architectural grading system. Gynecol Oncol. 1998. 70:2–12.
Article21. Mayr D, Diebold J. Grading of ovarian carcinomas. Int J Gynecol Pathol. 2000. 19:348–353.
Article22. Sato Y, Shimamoto T, Amada S, Asada Y, Hayashi T. Prognostic value of histologic grading of ovarian carcinomas. Int J Gynecol Pathol. 2003. 22:52–56.
Article23. Gershenson DM, Sun CC, Lu KH, Coleman RL, Sood AK, Malpica A, et al. Clinical behavior of stage II-IV low-grade serous carcinoma of the ovary. Obstet Gynecol. 2006. 108:361–368.
Article24. McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996. 334:1–6.
Article25. Muggia FM, Braly PS, Brady MF, Sutton G, Niemann TH, Lentz SL, et al. Phase III randomized study of cisplatin versus paclitaxel versus cisplatin and paclitaxel in patients with suboptimal stage III or IV ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2000. 18:106–115.
Article26. Plaxe SC. Epidemiology of low-grade serous ovarian cancer. Am J Obstet Gynecol. 2008. 198:459.e1–459.e8. discussion 459.e8-9.
Article27. Mayo LD, Donner DB. The PTEN, Mdm2, p53 tumor suppressor-oncoprotein network. Trends Biochem Sci. 2002. 27:462–467.
Article28. Kohler MF, Kerns BJ, Humphrey PA, Marks JR, Bast RC Jr, Berchuck A. Mutation and overexpression of p53 in early-stage epithelial ovarian cancer. Obstet Gynecol. 1993. 81:643–650.29. Kupryjańczyk J, Thor AD, Beauchamp R, Merritt V, Edgerton SM, Bell DA, et al. p53 gene mutations and protein accumulation in human ovarian cancer. Proc Natl Acad Sci U S A. 1993. 90:4961–4965.
Article30. Kurman RJ, Shih IeM. Pathogenesis of ovarian cancer: lessons from morphology and molecular biology and their clinical implications. Int J Gynecol Pathol. 2008. 27:151–160.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Targeting BRAF pathway in low-grade serous ovarian cancer
- Paired Primary and Metastatic Tumor Analysis of Somatic Mutations in Synchronous and Metachronous Colorectal Cancer
- Clinical Impact of Somatic Variants in Homologous RecombinationRepair-Related Genes in Ovarian High-Grade Serous Carcinoma
- Expression and Mutational Analysis of c-kit in Ovarian Surface Epithelial Tumors
- KRAS, NRAS, and BRAF mutations in plasma cell myeloma at a single Korean institute