Cancer Res Treat.
2017 Oct;49(4):898-905. 10.4143/crt.2016.312.
Effect of Adjuvant Chemotherapy after Complete Resection for Pathologic Stage IB Lung Adenocarcinoma in High-Risk Patients as Defined by a New Recurrence Risk Scoring Model
- Affiliations
-
- 1Department of Thoracic and Cardiovascular Surgery, Seoul National University Bundang Hospital, Seongnam, Korea. skcho@snubh.org
- 2Department of Thoracic and Cardiovascular Surgery, Seoul National University College of Medicine, Seoul, Korea.
- 3Research Institute and Hospital, National Cancer Center, Goyang, Korea.
- 4Department of Thoracic and Cardiovascular Surgery, Asan Medical Center, Seoul, Korea.
- KMID: 2394809
- DOI: http://doi.org/10.4143/crt.2016.312
Abstract
- PURPOSE
We conducted a retrospective analysis to determine if adjuvant chemotherapy prolongs overall survival in patients with pathologic stage IB lung adenocarcinoma who had undergone complete resection and were defined as high-risk by a newly developed recurrence risk scoring model.
MATERIALS AND METHODS
Patients who underwent curative resection for stage IB lung adenocarcinoma were analyzed with a newly developed recurrence risk scoring model and divided into a low-risk group and a high-risk group. The patients in the high-risk group were retrospectively divided into two groups based on whether they underwent adjuvant chemotherapy or observation. Recurrence-free survival and overall survival were compared between these two groups.
RESULTS
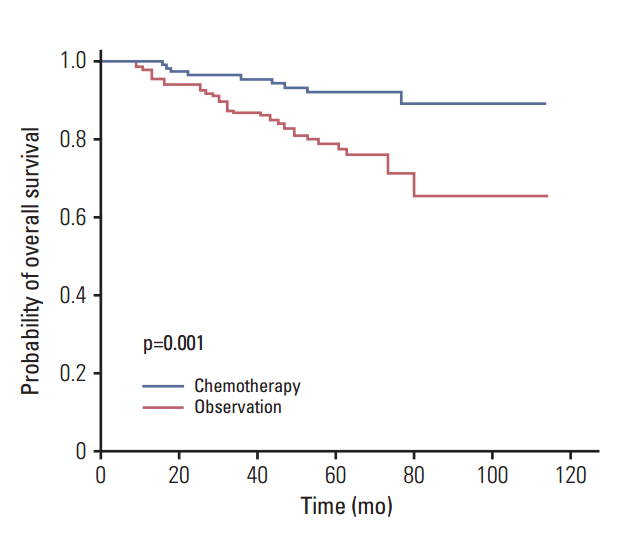
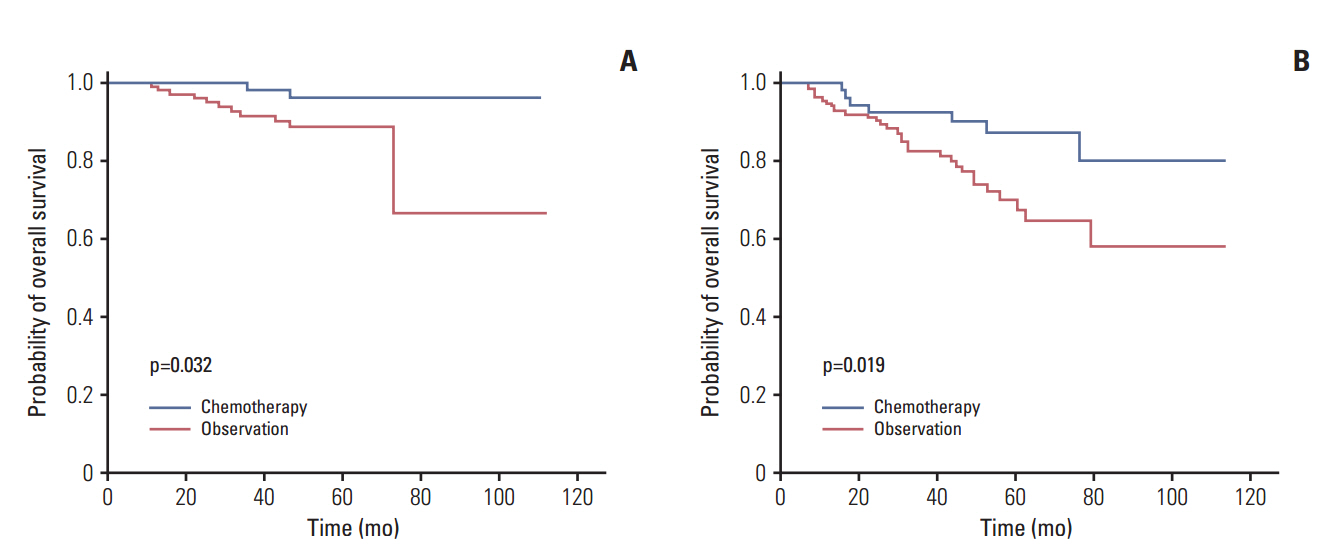
A total of 328 patients who underwent curative resection between 2000 and 2009 were included in this study, of whom 110 (34%) received adjuvant chemotherapy and 218 (67%) underwent observation without additional treatment. According to our risk model, 167 patients (51%) were high-risk and 161 (49%) were low-risk. The 5-year recurrence-free survival rates and overall survival were 84.4% and 91.5% in low-risk patients and 53.9% and 74.7% in high-risk patients (p < 0.001). In high-risk patients, the 5-year overall survival rates were 77% among patients who underwent observation and 87% among those who underwent adjuvant chemotherapy (p=0.019).
CONCLUSION
Adjuvant chemotherapy prolonged overall survival among high-risk patients who had undergone complete resection for stage IB lung adenocarcinoma.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
Article2. Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007; 2:706–14.
Article3. Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004; 350:351–60.
Article4. Douillard JY, Rosell R, De Lena M, Carpagnano F, Ramlau R, Gonzales-Larriba JL, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006; 7:719–27.
Article5. Winton T, Livingston R, Johnson D, Rigas J, Johnston M, Butts C, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005; 352:2589–97.
Article6. Strauss GM, Herndon JE 2nd, Maddaus MA, Johnstone DW, Johnson EA, Harpole DH, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol. 2008; 26:5043–51.
Article7. Carlson RW, Larsen JK, McClure J, Fitzgerald CL, Venook AP, Benson AB 3rd, et al. International adaptations of NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2014; 12:643–8.
Article8. Yang HC, Kim HR, Jheon S, Kim K, Cho S, Ahn S, et al. Recurrence risk-scoring model for stage I adenocarcinoma of the lung. Ann Surg Oncol. 2015; 22:4089–97.
Article9. Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovascular Surg. 1975; 70:606–12.
Article10. Ramnath N, Dilling TJ, Harris LJ, Kim AW, Michaud GC, Balekian AA, et al. Treatment of stage III non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013; 143(5 Suppl):e314S.11. Scagliotti GV, Fossati R, Torri V, Crino L, Giaccone G, Silvano G, et al. Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small-cell lung cancer. J Natl Cancer Inst. 2003; 95:1453–61.
Article12. Shimizu K, Yoshida J, Nagai K, Nishimura M, Ishii G, Morishita Y, et al. Visceral pleural invasion is an invasive and aggressive indicator of non-small cell lung cancer. J Thorac Cardiovasc Surg. 2005; 130:160–5.
Article13. Mizuno T, Ishii G, Nagai K, Yoshida J, Nishimura M, Mochizuki T, et al. Identification of a low risk subgroup of stage IB lung adenocarcinoma patients. Lung Cancer. 2008; 62:302–8.
Article14. Nitadori J, Colovos C, Kadota K, Sima CS, Sarkaria IS, Rizk NP, et al. Visceral pleural invasion does not affect recurrence or overall survival among patients with lung adenocarcinoma ≤ 2 cm: a proposal to reclassify T1 lung adenocarcinoma. Chest. 2013; 144:1622–31.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The solid predominant subtype as an independent risk factor for recurrence in patients with pathologic stage I lung adenocarcinoma
- Risk Factor for Recurrence in Completely Resected Stage IB Non-small Cell Lung Cancer
- Risk-adapted scoring model to identify candidates benefiting from adjuvant chemotherapy after radical nephroureterectomy for localized upper urinary tract urothelial carcinoma: A multicenter study
- Prognostic Significance of Claudin 4 in Completely Resected Adenocarcinoma of the Lung
- Neoadjuvant and postoperative chemotherapy with paclitaxel plus cisplatin for the treatment of FIGO stage IB cervical cancer in pregnancy