J Vet Sci.
2012 Dec;13(4):331-338.
Prolonged expression of senescence markers in mice exposed to gamma-irradiation
- Affiliations
-
- 1Radiation Biotechnology Research Division, Advanced Radiation Technology Institute, Korea Atomic Energy Research Institute, Jeongeup 580-185, Korea. uhjung@kaeri.re.kr
Abstract
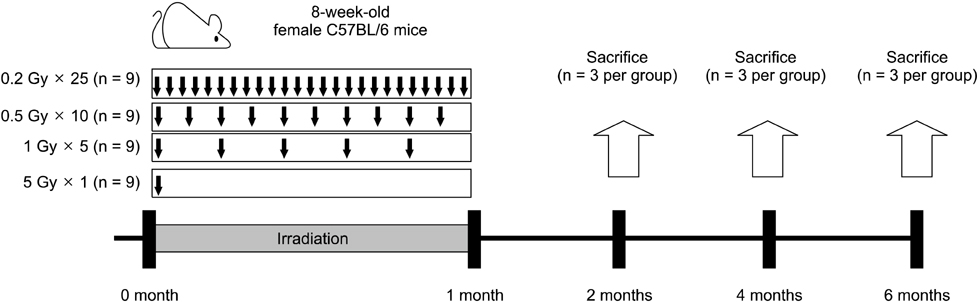
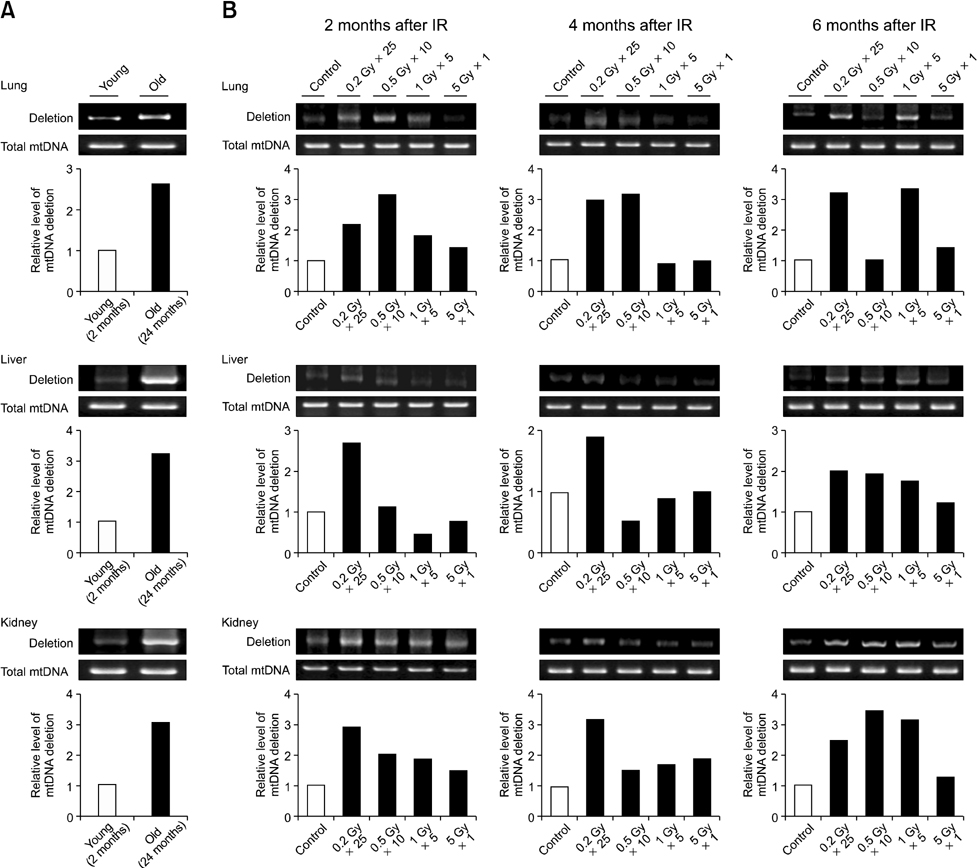
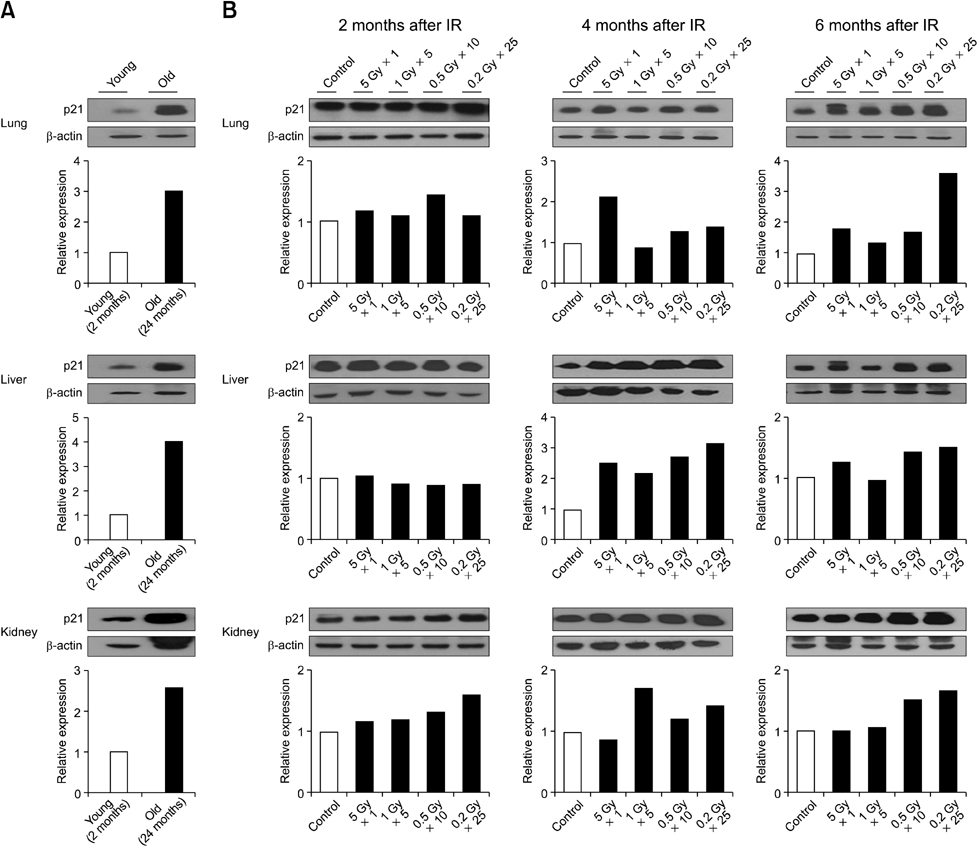
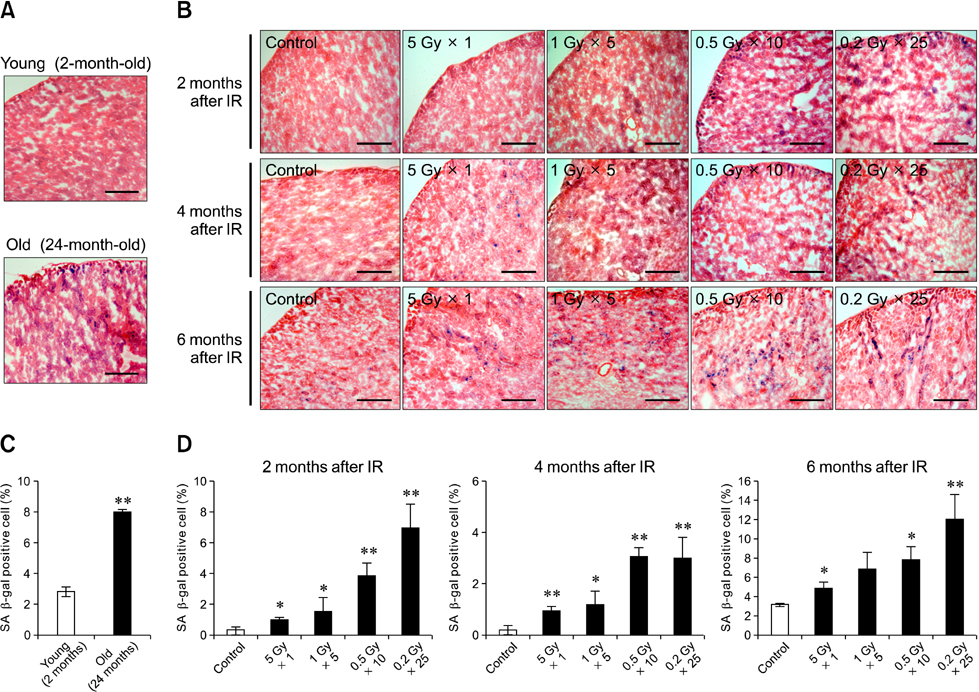
- Although ionizing radiation is known to induce cellular senescence in vitro and in vivo, its long-term in vivo effects are not well defined. In this study, we examined the prolonged expression of senescence markers in mice irradiated with single or fractionated doses. C57BL/6 female mice were exposed to 5 Gy of gamma-rays in single or 5, 10, 25 fractions. At 2, 4, and 6 months after irradiation, senescence markers including mitochondrial DNA (mtDNA) common deletion, p21, and senescence-associated beta-galactosidase (SA beta-gal) were monitored in the lung, liver, and kidney. Increases of mtDNA deletion were detected in the lung, liver, and kidney of irradiated groups. p21 expression and SA beta-gal staining were also increased in the irradiated groups compared to the non-irradiated control group. Increases of senescence markers persisted up to 6 months after irradiation. Additionally, the extent of mtDNA deletion and the numbers of SA beta-gal positive cells were greater as the number of radiation fractions increased. In conclusion, our results showed that ionizing radiation, especially that delivered in fractions, can cause the persistent upregulation of senescence marker expression in vivo. This should be considered when dealing with chronic normal tissue injuries caused by radiation therapy or radiation accidents.
Keyword
MeSH Terms
Figure
Reference
-
1. Barsyte D, Lovejoy DA, Lithgow GJ. Longevity and heavy metal resistance in daf-2 and age-1 long-lived mutants of Caenorhabditis elegans. FASEB J. 2001. 15:627–634.
Article2. Berneburg M, Plettenberg H, Medve-König K, Pfahlberg A, Gers-Barlag H, Gefeller O, Krutmann J. Induction of the photoaging-associated mitochondrial common deletion in vivo in normal human skin. J Invest Dermatol. 2004. 122:1277–1283.
Article3. Choi J, Shendrik I, Peacocke M, Peehl D, Buttyan R, Ikeguchi EF, Katz AE, Benson MC. Expression of senescence-associated beta-galactosidase in enlarged prostates from men with benign prostatic hyperplasia. Urology. 2000. 56:160–166.
Article4. Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001. 292:104–106.
Article5. Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, Peacocke M, Campisi J. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995. 92:9363–9367.
Article6. Eriksson D, Stigbrand T. Radiation-induced cell death mechanisms. Tumour Biol. 2010. 31:363–372.
Article7. Gresh L, Fischer E, Reimann A, Tanguy M, Garbay S, Shao X, Hiesberger T, Fiette L, Igarashi P, Yaniv M, Pontoglio M. A transcriptional network in polycystic kidney disease. EMBO J. 2004. 23:1657–1668.
Article8. Han YH, Kim HS, Kim JM, Kim SK, Yu DY, Moon EY. Inhibitory role of peroxiredoxin II (Prx II) on cellular senescence. FEBS Lett. 2005. 579:4897–4902.
Article9. Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in Ames dwarf mice: correlation to extended longevity. J Gerontol A Biol Sci Med Sci. 2003. 58:291–296.
Article10. Jackson JG, Post SM, Lozano G. Regulation of tissue- and stimulus-specific cell fate decisions by p53 in vivo. J Pathol. 2011. 223:127–136.11. Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC, Sahin E, Kost-Alimova M, Protopopov A, Cadiñanos J, Horner JW, Maratos-Flier E, Depinho RA. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011. 469:102–106.
Article12. Jeyapalan JC, Sedivy JM. Cellular senescence and organismal aging. Mech Ageing Dev. 2008. 129:467–474.
Article13. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002. 418:41–49.
Article14. Jo SK, Seol MA, Park HR, Jung U, Roh C. Ionising radiation triggers fat accumulation in white adipose tissue. Int J Radiat Biol. 2011. 87:302–310.
Article15. Kailong L, Du X, Yani H, Lin Z, Jvrong Y, Ruihua S, Lin C. p53-Rb signaling pathway is involved in tubular cell senescence in renal ischemia/reperfusion injury. Biocell. 2007. 31:213–223.
Article16. Le ONL, Rodier F, Fontaine F, Coppe JP, Campisi J, DeGregori J, Laverdière C, Kokta V, Haddad E, Beauséjour CM. Ionizing radiation-induced long-term expression of senescence markers in mice is independent of p53 and immune status. Aging Cell. 2010. 9:398–409.
Article17. Ling CC, Guo M, Chen CH, Deloherey T. Radiation-induced apoptosis: effects of cell age and dose fractionation. Cancer Res. 1995. 55:5207–5212.18. Little MP. Cancer and non-cancer effects in Japanese atomic bomb survivors. J Radiol Prot. 2009. 29:A43–A59.
Article19. Maruyama N, Ishigami A, Kuramoto M, Handa S, Kubo S, Imasawa T, Seyama K, Shimosawa T, Kasahara Y. Senescence marker protein-30 knockout mouse as an aging model. Ann N Y Acad Sci. 2004. 1019:383–387.
Article20. Meng A, Wang Y, Van Zant G, Zhou D. Ionizing radiation and busulfan induce premature senescence in murine bone marrow hematopoietic cells. Cancer Res. 2003. 63:5414–5419.21. Mirzayans R, Andrais B, Scott A, Paterson MC, Murray D. Single-cell analysis of p16INK4a and p21WAF1 expression suggests distinct mechanisms of senescence in normal human and Li-Fraumeni syndrome fibroblasts. J Cell Physiol. 2010. 223:57–67.22. Murakami S. Stress resistance in long-lived mouse models. Exp Gerontol. 2006. 41:1014–1019.
Article23. Muthna D, Soukup T, Vavrova J, Mokry J, Cmielova J, Visek B, Jiroutova A, Havelek R, Suchanek J, Filip S, English D, Rezacova M. Irradiation of adult human dental pulp stem cells provokes activation of p53, cell cycle arrest, and senescence but not apoptosis. Stem Cells Dev. 2010. 19:1855–1862.
Article24. Oh CW, Bump EA, Kim JS, Janigro D, Mayberg MR. Induction of a senescence-like phenotype in bovine aortic endothelial cells by ionizing radiation. Radiat Res. 2001. 156:232–240.
Article25. Park HR, Jo SK, Eom HS. Chronic effects of single and fractionated γ-irradiation on an impairment of Th1-related immune response. Int J Radiat Biol. 2011. 87:534–543.
Article26. Prithivirajsingh S, Story MD, Bergh SA, Geara FB, Ang KK, Ismail SM, Stevens CW, Buchholz TA, Brock WA. Accumulation of the common mitochondrial DNA deletion induced by ionizing radiation. FEBS Lett. 2004. 571:227–232.
Article27. Richardson RB. Ionizing radiation and aging: rejuvenating an old idea. Aging (Albany NY). 2009. 1:887–902.
Article28. Rodier F, Coppé JP, Patil CK, Hoeijmakers WAM, Muñoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009. 11:973–979.
Article29. Sabin RJ, Anderson RM. Cellular senescence - its role in cancer and the response to ionizing radiation. Genome Integr. 2011. 2:7.
Article30. Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010. 464:520–528.
Article31. Severino J, Allen RG, Balin S, Balin A, Cristofalo VJ. Is β-galactosidase staining a marker of senescence in vitro and in vivo? Exp Cell Res. 2000. 257:162–171.32. Suh Y. Cell signaling in aging and apoptosis. Mech Ageing Dev. 2002. 123:881–890.
Article33. Vergara M, Smith-Wheelock M, Harper JM, Sigler R, Miller RA. Hormone-treated Snell dwarf mice regain fertility but remain long lived and disease resistant. J Gerontol A Biol Sci Med Sci. 2004. 59:1244–1250.
Article34. Wang C, Jurk D, Maddick M, Nelson G, Martin-Ruiz C, von Zglinicki T. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell. 2009. 8:311–323.
Article35. Wang L, Kuwahara Y, Li L, Baba T, Shin RW, Ohkubo Y, Ono K, Fukumoto M. Analysis of common deletion (CD) and a novel deletion of mitochondrial DNA induced by ionizing radiation. Int J Radiat Biol. 2007. 83:433–442.
Article36. Wang Y, Schulte BA, LaRue AC, Ogawa M, Zhou D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood. 2006. 107:358–366.
Article37. Zhan H, Suzuki T, Aizawa K, Miyagawa K, Nagai R. Ataxia telangiectasia mutated (ATM)-mediated DNA damage response in oxidative stress-induced vascular endothelial cell senescence. J Biol Chem. 2010. 285:29662–29670.
Article38. Zhang X, Han D, Ding D, Dai P, Yang W, Jiang S, Salvi RJ. Cochlear mitochondrial DNA3867bp deletion in aged mice. Chin Med J (Engl). 2002. 115:1390–1393.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Thermogenesis and cellular senescence of diabetic adipocytes in response to β-agonists and 18-carbon fatty acids
- Expression of Inhibin in the Whole-body gamma-irradiated Mouse Ovary
- Ultraviolet B-induced Senescence Model Using Corneal Fibroblasts and the Anti-aging Effect of Angiogenin
- The Effect of High Dose UVA - 1 and UVA - 2 Irradiation on the Expression of Surface Markers of Epidermal Langerhans Cells and Induction of contact Hypersensitivity in Mice Skin
- Comparison of Resistance to gamma-Irradiation between Cryptosporidium parvum and Cryptosporidium muris Using In Vivo Infection