Clin Exp Vaccine Res.
2013 Jan;2(1):69-70.
Clinical trials for vaccine development in registry of Korea Food and Drug Administration
- Affiliations
-
- 1Biologics Division, Korea Food and Drug Administration, Cheongwon, Korea. ksy1205@kfda.go.kr
Abstract
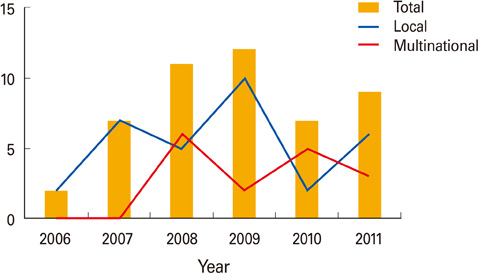
- Based on the action plan "Ensuring a stable supply of National Immunization Program vaccines and sovereignty of biopharmaceutical products," Korea Food and Drug Administration (KFDA) has made efforts to develop vaccines in the context of self reliance and to protect public health. Along with the recognized infrastructures for clinical trials, clinical trials for vaccines have also gradually been conducted at multinational sites as well as at local sites. KFDA will support to expand six to eleven kinds of vaccines by 2017. In accordance with integrated regulatory system, KFDA has promoted clinical trials, established national lot release procedure, and strengthened good manufacturing practices inspection and post marketing surveillance. Against this backdrop, KFDA will support the vaccine development and promote excellent public health protection.
MeSH Terms
Figure
Reference
-
1. Korea Food and Drug Administration. Food and drug statistical yearbook, 2011. 2011. Cheongwon: Korea Food and Drug Administration.2. Korea Food and Drug Administration. Food and drug statistical yearbook, 2011 [Internet]. c2012. cited 2012 Nov 5. Cheongwon: Korea Food and Drug Administration;Available from: http://www.kfda.go.kr/index.kfda?mid=96&pageNo=1&seq=12217&cmd=v.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current status of registry of vaccine clinical trials conducted by Korean investigators in ClinicalTrials.gov, database of US National Institutes of Health
- Trends of clinical trials from 2014 to 2016 in South Korea
- Genetically Engineered Mouse Models for Drug Development and Preclinical Trials
- Regulations and Guidelines for Planning and Design of Multi-regional Clinical Trials
- Trends of clinical trials from 2017 to 2019 in Korea: an integrated analysis based on the Ministry of Food and Drug Safety (MFDS) and the Clinical Research Information Service (CRIS) registries