J Bone Metab.
2014 Feb;21(1):41-54.
Role of Osteocyte-derived Insulin-Like Growth Factor I in Developmental Growth, Modeling, Remodeling, and Regeneration of the Bone
- Affiliations
-
- 1Division of Regenerative Medicine, Department of Medicine, Loma Linda University School of Medicine, Loma Linda, CA, USA. Dbaylink@llu.edu
- 2Musculoskeletal Disease Center, Jerry L. Pettis Memorial VA Medical Center, Loma Linda, CA, USA.
Abstract
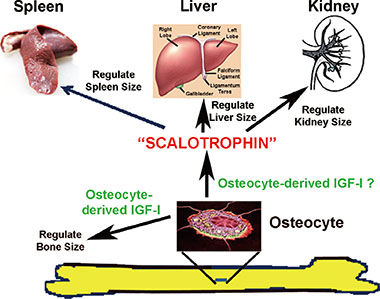
- The osteocyte has long been considered to be the primary mechanosensory cell in the bone. Recent evidence has emerged that the osteocyte is also a key regulator of various bone and mineral metabolism and that its regulatory effects are in part mediated through locally produced osteocyte-derived factors, such as sclerostin, receptor activator of nuclear factor-kappa B ligand (RANKL), and fibroblast growth factor (FGF)-23. Osteocytes secrete large amounts of insulin-like growth factor (IGF)-I in bone. Although IGF-I produced locally by other bone cells, such as osteoblasts and chondrocytes, has been shown to play important regulatory roles in bone turnover and developmental bone growth, the functional role of osteocyte-derived IGF-I in the bone and mineral metabolism has not been investigated and remains unclear. However, results of recent studies in osteocyte Igf1 conditional knockout transgenic mice have suggested potential regulatory roles of osteocyte-derived IGF-I in various aspects of bone and mineral metabolism. In this review, evidence supporting a regulatory role for osteocyte-derived IGF-I in the osteogenic response to mechanical loading, the developmental bone growth, the bone response to dietary calcium depletion and repletion, and in fracture repair is discussed. A potential coordinated regulatory relationship between the effect of osteocyte-derived IGF-I on bone size and the internal organ size is also proposed.
Keyword
MeSH Terms
-
Animals
Bone Development
Bone Regeneration
Bone Remodeling
Calcium, Dietary
Chondrocytes
Fibroblast Growth Factors
Fracture Healing
Insulin-Like Growth Factor I*
Metabolism
Mice
Mice, Transgenic
Organ Size
Osteoblasts
Osteocytes
RANK Ligand
Regeneration*
Calcium, Dietary
Fibroblast Growth Factors
Insulin-Like Growth Factor I
RANK Ligand
Figure
Reference
-
1. Kimmel DB. A paradigm for skeletal strength homeostasis. J Bone Miner Res. 1993; 8:Suppl 2. S515–S522.
Article2. Kobayashi S, Takahashi HE, Ito A, et al. Trabecular minimodeling in human iliac bone. Bone. 2003; 32:163–169.3. Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000; 21:115–137.
Article4. Atkins GJ, Findlay DM. Osteocyte regulation of bone mineral: a little give and take. Osteoporos Int. 2012; 23:2067–2079.
Article5. Polig E, Jee WS. A model of osteon closure in cortical bone. Calcif Tissue Int. 1990; 47:261–269.
Article6. Franz-Odendaal TA, Hall BK, Witten PE. Buried alive: how osteoblasts become osteocytes. Dev Dyn. 2006; 235:176–190.
Article7. Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011; 26:229–238.
Article8. Winkler DG, Sutherland MK, Geoghegan JC, et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003; 22:6267–6276.
Article9. Nakashima T, Hayashi M, Fukunaga T, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011; 17:1231–1234.
Article10. Xiong J, Onal M, Jilka RL, et al. Matrix-embedded cells control osteoclast formation. Nat Med. 2011; 17:1235–1241.
Article11. Bonewald LF. Osteocytes as dynamic multifunctional cells. Ann N Y Acad Sci. 2007; 1116:281–290.
Article12. Feng JQ, Clinkenbeard EL, Yuan B, et al. Osteocyte regulation of phosphate homeostasis and bone mineralization underlies the pathophysiology of the heritable disorders of rickets and osteomalacia. Bone. 2013; 54:213–221.
Article13. Quarles LD. Skeletal secretion of FGF-23 regulates phosphate and vitamin D metabolism. Nat Rev Endocrinol. 2012; 8:276–286.
Article14. Mohan S, Baylink DJ. Bone growth factors. Clin Orthop Relat Res. 1991; 30–48.
Article15. McCarthy TL, Centrella M, Canalis E. Insulin-like growth factor (IGF) and bone. Connect Tissue Res. 1989; 20:277–282.
Article16. Grinspoon SK, Baum HB, Peterson S, et al. Effects of rhIGF-I administration on bone turnover during short-term fasting. J Clin Invest. 1995; 96:900–906.
Article17. Behringer RR, Lewin TM, Quaife CJ, et al. Expression of insulin-like growth factor I stimulates normal somatic growth in growth hormone-deficient transgenic mice. Endocrinology. 1990; 127:1033–1040.
Article18. Mohan S, Richman C, Guo R, et al. Insulin-like growth factor regulates peak bone mineral density in mice by both growth hormone-dependent and -independent mechanisms. Endocrinology. 2003; 144:929–936.
Article19. Wang Y, Nishida S, Elalieh HZ, et al. Role of IGF-I signaling in regulating osteoclastogenesis. J Bone Miner Res. 2006; 21:1350–1358.
Article20. Lean JM, Mackay AG, Chow JW, et al. Osteocytic expression of mRNA for c-fos and IGF-I: an immediate early gene response to an osteogenic stimulus. Am J Physiol. 1996; 270:E937–E945.
Article21. Sjögren K, Liu JL, Blad K, et al. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci U S A. 1999; 96:7088–7092.
Article22. Govoni KE, Wergedal JE, Florin L, et al. Conditional deletion of insulin-like growth factor-I in collagen type 1alpha2-expressing cells results in postnatal lethality and a dramatic reduction in bone accretion. Endocrinology. 2007; 148:5706–5715.
Article23. Govoni KE, Lee SK, Chung YS, et al. Disruption of insulin-like growth factor-I expression in type IIalphaI collagen-expressing cells reduces bone length and width in mice. Physiol Genomics. 2007; 30:354–362.
Article24. Turner CH. Bone strength: current concepts. Ann N Y Acad Sci. 2006; 1068:429–446.
Article25. Hara M, Tabata K, Suzuki T, et al. Calcium influx through a possible coupling of cation channels impacts skeletal muscle satellite cell activation in response to mechanical stretch. Am J Physiol Cell Physiol. 2012; 302:C1741–C1750.
Article26. Ishihara Y, Sugawara Y, Kamioka H, et al. Ex vivo real-time observation of Ca(2+) signaling in living bone in response to shear stress applied on the bone surface. Bone. 2013; 53:204–215.
Article27. Soria B, Navas S, Hmadcha A, et al. Single mechanosensitive and Ca(2)(+)-sensitive channel currents recorded from mouse and human embryonic stem cells. J Membr Biol. 2013; 246:215–230.
Article28. Gaston J, Quinchia Rios B, Bartlett R, et al. The response of vocal fold fibroblasts and mesenchymal stromal cells to vibration. PLoS One. 2012; 7:e30965.
Article29. Kamioka H, Ishihara Y, Ris H, et al. Primary cultures of chick osteocytes retain functional gap junctions between osteocytes and between osteocytes and osteoblasts. Microsc Microanal. 2007; 13:108–117.
Article30. Kamioka H, Honjo T, Takano-Yamamoto T. A three-dimensional distribution of osteocyte processes revealed by the combination of confocal laser scanning microscopy and differential interference contrast microscopy. Bone. 2001; 28:145–149.
Article31. Tatsumi S, Ishii K, Amizuka N, et al. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007; 5:464–475.
Article32. Kapur S, Baylink DJ, Lau KH. Fluid flow shear stress stimulates human osteoblast proliferation and differentiation through multiple interacting and competing signal transduction pathways. Bone. 2003; 32:241–251.
Article33. Lau KH, Kapur S, Kesavan C, et al. Up-regulation of the Wnt, estrogen receptor, insulin-like growth factor-I, and bone morphogenetic protein pathways in C57BL/6J osteoblasts as opposed to C3H/HeJ osteoblasts in part contributes to the differential anabolic response to fluid shear. J Biol Chem. 2006; 281:9576–9588.
Article34. Pavalko FM, Chen NX, Turner CH, et al. Fluid shear-induced mechanical signaling in MC3T3-E1 osteoblasts requires cytoskeleton-integrin interactions. Am J Physiol. 1998; 275:C1591–C1601.35. Temiyasathit S, Tang WJ, Leucht P, et al. Mechanosensing by the primary cilium: deletion of Kif3A reduces bone formation due to loading. PLoS One. 2012; 7:e33368.
Article36. Xiao Z, Dallas M, Qiu N, et al. Conditional deletion of Pkd1 in osteocytes disrupts skeletal mechanosensing in mice. FASEB J. 2011; 25:2418–2432.
Article37. Zhou H, Newnum AB, Martin JR, et al. Osteoblast/osteocyte-specific inactivation of Stat3 decreases load-driven bone formation and accumulates reactive oxygen species. Bone. 2011; 49:404–411.
Article38. Grimston SK, Goldberg DB, Watkins M, et al. Connexin43 deficiency reduces the sensitivity of cortical bone to the effects of muscle paralysis. J Bone Miner Res. 2011; 26:2151–2160.
Article39. Kawata A, Mikuni-Takagaki Y. Mechanotransduction in stretched osteocytes--temporal expression of immediate early and other genes. Biochem Biophys Res Commun. 1998; 246:404–408.
Article40. Reijnders CM, Bravenboer N, Tromp AM, et al. Effect of mechanical loading on insulin-like growth factor-I gene expression in rat tibia. J Endocrinol. 2007; 192:131–140.
Article41. Gross TS, Srinivasan S, Liu CC, et al. Noninvasive loading of the murine tibia: an in vivo model for the study of mechanotransduction. J Bone Miner Res. 2002; 17:493–501.
Article42. Sakata T, Halloran BP, Elalieh HZ, et al. Skeletal unloading induces resistance to insulin-like growth factor I on bone formation. Bone. 2003; 32:669–680.
Article43. Sakata T, Wang Y, Halloran BP, et al. Skeletal unloading induces resistance to insulin-like growth factor-I (IGF-I) by inhibiting activation of the IGF-I signaling pathways. J Bone Miner Res. 2004; 19:436–446.
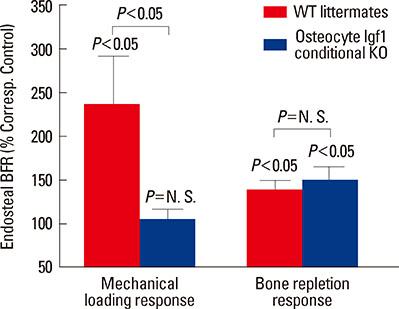
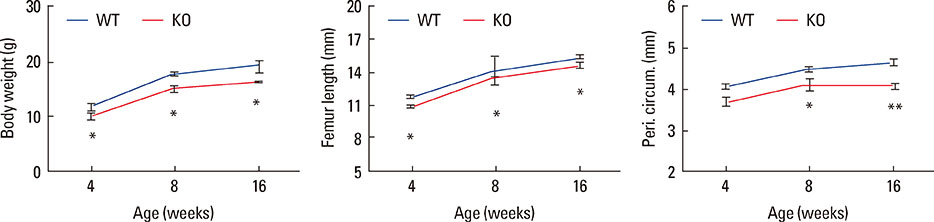
Article44. Kesavan C, Wergedal JE, Lau KH, et al. Conditional disruption of IGF-I gene in type 1alpha collagen-expressing cells shows an essential role of IGF-I in skeletal anabolic response to loading. Am J Physiol Endocrinol Metab. 2011; 301:E1191–E1197.45. Lau KH, Baylink DJ, Zhou XD, et al. Osteocyte-derived insulin-like growth factor I is essential for determining bone mechanosensitivity. Am J Physiol Endocrinol Metab. 2013; 305:E271–E281.46. Sheng MH, Zhou XD, Bonewald LF, et al. Disruption of the insulin-like growth factor-1 gene in osteocytes impairs developmental bone growth in mice. Bone. 2013; 52:133–144.
Article47. Olsen BR, Reginato AM, Wang W. Bone development. Annu Rev Cell Dev Biol. 2000; 16:191–220.
Article48. Ahlborg HG, Johnell O, Turner CH, et al. Bone loss and bone size after menopause. N Engl J Med. 2003; 349:327–334.
Article49. Orwoll ES. Toward an expanded understanding of the role of the periosteum in skeletal health. J Bone Miner Res. 2003; 18:949–954.
Article50. Libanati C, Baylink DJ, Lois-Wenzel E, et al. Studies on the potential mediators of skeletal changes occurring during puberty in girls. J Clin Endocrinol Metab. 1999; 84:2807–2814.
Article51. Heaney RP, Abrams S, Dawson-Hughes B, et al. Peak bone mass. Osteoporos Int. 2000; 11:985–1009.
Article52. van der Eerden BC, Karperien M, Wit JM. Systemic and local regulation of the growth plate. Endocr Rev. 2003; 24:782–801.
Article53. Mackie EJ, Tatarczuch L, Mirams M. The skeleton: a multi-functional complex organ: the growth plate chondrocyte and endochondral ossification. J Endocrinol. 2011; 211:109–121.
Article54. Ohlsson C, Mohan S, Sjögren K, et al. The role of liver-derived insulin-like growth factor-I. Endocr Rev. 2009; 30:494–535.
Article55. Olson LE, Ohlsson C, Mohan S. The role of GH/IGF-I-mediated mechanisms in sex differences in cortical bone size in mice. Calcif Tissue Int. 2011; 88:1–8.
Article56. Mohan S, Baylink DJ. Serum insulin-like growth factor binding protein (IGFBP)-4 and IGFBP-5 levels in aging and age-associated diseases. Endocrine. 1997; 7:87–91.
Article57. Yamada PM, Lee KW. Perspectives in mammalian IGFBP-3 biology: local vs. systemic action. Am J Physiol Cell Physiol. 2009; 296:C954–C976.
Article58. Zhang P, Yokota H. Elbow loading promotes longitudinal bone growth of the ulna and the humerus. J Bone Miner Metab. 2012; 30:31–39.
Article59. Li X, Zhang Y, Kang H, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005; 280:19883–19887.
Article60. Wijenayaka AR, Kogawa M, Lim HP, et al. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS One. 2011; 6:e25900.
Article61. Poole KE, van Bezooijen RL, Loveridge N, et al. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005; 19:1842–1844.
Article62. Collins JN, Kirby BJ, Woodrow JP, et al. Lactating Ctcgrp nulls lose twice the normal bone mineral content due to fewer osteoblasts and more osteoclasts, whereas bone mass is fully restored after weaning in association with up-regulation of Wnt signaling and other novel genes. Endocrinology. 2013; 154:1400–1413.
Article63. Bèlanger LF. Osteocytic osteolysis. Calcif Tissue Res. 1969; 4:1–12.
Article64. Parfitt AM. The cellular basis of bone turnover and bone loss: a rebuttal of the osteocytic resorption--bone flow theory. Clin Orthop Relat Res. 1977; 236–247.65. Boyde A. Evidence against osteocytic osteolysis. Metab Bone Dis Relat Res. 1980; 2S:239–255.66. Qing H, Ardeshirpour L, Pajevic PD, et al. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J Bone Miner Res. 2012; 27:1018–1029.
Article67. Tazawa K, Hoshi K, Kawamoto S, et al. Osteocytic osteolysis observed in rats to which parathyroid hormone was continuously administered. J Bone Miner Metab. 2004; 22:524–529.
Article68. Qing H, Bonewald LF. Osteocyte remodeling of the perilacunar and pericanalicular matrix. Int J Oral Sci. 2009; 1:59–65.
Article69. Kogawa M, Wijenayaka AR, Ormsby RT, et al. Sclerostin regulates release of bone mineral by osteocytes by induction of carbonic anhydrase 2. J Bone Miner Res. 2013; 28:2436–2448.
Article70. Bikle DD, Wang Y. Insulin like growth factor-I: a critical mediator of the skeletal response to parathyroid hormone. Curr Mol Pharmacol. 2012; 5:135–142.
Article71. Rhee Y, Allen MR, Condon K, et al. PTH receptor signaling in osteocytes governs periosteal bone formation and intracortical remodeling. J Bone Miner Res. 2011; 26:1035–1046.
Article72. Saini V, Marengi DA, Barry KJ, et al. Parathyroid hormone (PTH)/PTH-related peptide type 1 receptor (PPR) signaling in osteocytes regulates anabolic and catabolic skeletal responses to PTH. J Biol Chem. 2013; 288:20122–20134.
Article73. Sheng MH, Lau KH, Zhou XD, et al. Dichotomy in osteocyte regulation of anabolic action of mechanical loading challenge and that of bone repletion after a low dietary calcium challenge. In : ASBMR 2013 Annual Meeting; 2013 October 4-7; Baltimore Convention Center. Baltimore, MD: American Society for Bone and Mineral Research.74. Li C, Ominsky MS, Tan HL, et al. Increased callus mass and enhanced strength during fracture healing in mice lacking the sclerostin gene. Bone. 2011; 49:1178–1185.
Article75. McGee-Lawrence ME, Ryan ZC, Carpio LR, et al. Sclerostin deficient mice rapidly heal bone defects by activating beta-catenin and increasing intramembranous ossification. Biochem Biophys Res Commun. 2013; 441:886–890.
Article76. Caetano-Lopes J, Lopes A, Rodrigues A, et al. Upregulation of inflammatory genes and downregulation of sclerostin gene expression are key elements in the early phase of fragility fracture healing. PLoS One. 2011; 6:e16947.
Article77. Gamie Z, Korres N, Leonidou A, et al. Sclerostin monoclonal antibodies on bone metabolism and fracture healing. Expert Opin Investig Drugs. 2012; 21:1523–1534.
Article78. Virk MS, Alaee F, Tang H, et al. Systemic administration of sclerostin antibody enhances bone repair in a critical-sized femoral defect in a rat model. J Bone Joint Surg Am. 2013; 95:694–701.
Article79. Nakajima A, Shimoji N, Shiomi K, et al. Mechanisms for the enhancement of fracture healing in rats treated with intermittent low-dose human parathyroid hormone (1-34). J Bone Miner Res. 2002; 17:2038–2047.
Article80. Ellegaard M, Kringelbach T, Syberg S, et al. The effect of PTH (1-34) on fracture healing during different loading conditions. J Bone Miner Res. 2013; 28:2145–2155.
Article81. Meinel L, Zoidis E, Zapf J, et al. Localized insulin-like growth factor I delivery to enhance new bone formation. Bone. 2003; 33:660–672.
Article82. Schmidmaier G, Wildemann B, Heeger J, et al. Improvement of fracture healing by systemic administration of growth hormone and local application of insulin-like growth factor-1 and transforming growth factor-beta1. Bone. 2002; 31:165–172.
Article83. Augat P, Simon U, Liedert A, et al. Mechanics and mechano-biology of fracture healing in normal and osteoporotic bone. Osteoporos Int. 2005; 16:Suppl 2. S36–S43.
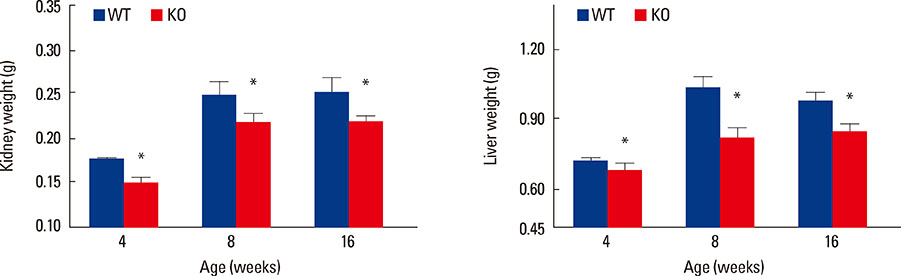
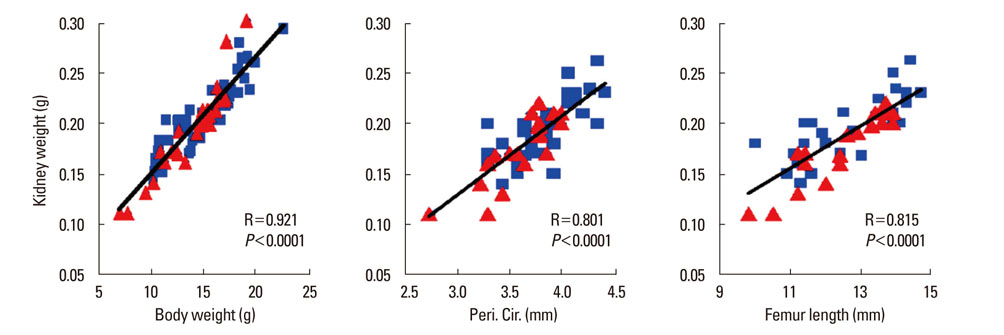
Article84. Ulstrup AK. Biomechanical concepts of fracture healing in weight-bearing long bones. Acta Orthop Belg. 2008; 74:291–302.85. Sheng MH, Zhou XD, Lau KH, et al. Conditional knockout of IGF-I in osteocytes surprisingly accelerates fracture repair. In : ASBMR 2011 Annual Meeting; 2011 September 16-20; San Diego Convention Center. San Diego, CA: American Society for Bone and Mineral Research.86. Rundle CH, Lau KH, Zhou XD, et al. Accelerated bony union during fracture healing in osteocyte-specific IGF-1 knockout mice. In : ORS 2013 Annual Meeting; 2011 January 26-29; Henry B. Gonzalez Convention Center. San Antonio, TX: Orthopaedic Research Society.87. Sheng MH, Zhou XD, Lau KH, et al. Evidence that osteocytes elaborate endocrine regulation of internal organs size: data from the osteocytes IGF-I conditional knockout mice. In : ASBMR 2011 Annual Meeting; San Diego Convention Center. San Diego, CA: American Society for Bone and Mineral Research;2011.88. Schafer AL, Sellmeyer DE, Schwartz AV, et al. Change in undercarboxylated osteocalcin is associated with changes in body weight, fat mass, and adiponectin: parathyroid hormone (1-84) or alendronate therapy in postmenopausal women with osteoporosis (the PaTH study). J Clin Endocrinol Metab. 2011; 96:E1982–E1989.
Article89. Barolo S, Posakony JW. Three habits of highly effective signaling pathways: principles of transcriptional control by developmental cell signaling. Genes Dev. 2002; 16:1167–1181.
Article90. Pan D. Hippo signaling in organ size control. Genes Dev. 2007; 21:886–897.
Article91. Camargo FD, Gokhale S, Johnnidis JB, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007; 17:2054–2060.
Article92. Dong J, Feldmann G, Huang J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007; 130:1120–1133.
Article93. Song H, Mak KK, Topol L, et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc Natl Acad Sci U S A. 2010; 107:1431–1436.
Article94. Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011; 13:877–883.
Article95. Schmidmaier G, Wildemann B, Gabelein T, et al. Synergistic effect of IGF-I and TGF-beta1 on fracture healing in rats: single versus combined application of IGF-I and TGF-beta1. Acta Orthop Scand. 2003; 74:604–610.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Relationship of Insulin like Growth Factor I with Pharmacologically Stimulated Growth Hormone Secretion in Growth Hormone Deficient Children
- The Role of the Insulin-like Growth Factors and Insulin-like Growth Factor Binding Proteins in Growth Disorders
- The Osteocyte Network as a Source and Reservoir of Signaling Factors
- Diabetes and Osteoporosis
- Recent Progress in Osteocyte Research