Clin Exp Vaccine Res.
2017 Jul;6(2):135-145. 10.7774/cevr.2017.6.2.135.
Preferential production of IgM-secreting hybridomas by immunization with DNA vaccines coding for Ebola virus glycoprotein: use of protein boosting for IgG-secreting hybridoma production
- Affiliations
-
- 1BK21 Plus Graduate Program and Department of Microbiology, School of Medicine, Kangwon National University, Chuncheon, Korea. jsin1964@hanmail.net
- KMID: 2391574
- DOI: http://doi.org/10.7774/cevr.2017.6.2.135
Abstract
- PURPOSE
The goal of this study was to investigate the utility of DNA vaccines encoding Ebola virus glycoprotein (GP) as a vaccine type for the production of GP-specific hybridomas and antibodies.
MATERIALS AND METHODS
DNA vaccines were constructed to express Ebola virus GP. Mice were injected with GP DNA vaccines and their splenocytes were used for hybridoma production. Enzyme-linked immunosorbent assays (ELISAs), limiting dilution subcloning, antibody purification methods, and Western blot assays were used to select GP-specific hybridomas and purify monoclonal antibodies (MAbs) from the hybridoma cells.
RESULTS
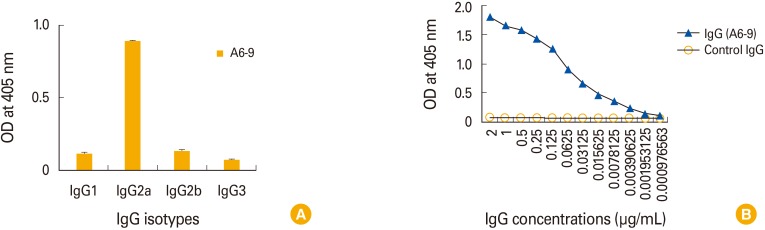
Twelve hybridomas, the cell supernatants of which displayed GP-binding activity, were selected by ELISA. When purified MAbs from 12 hybridomas were tested for their reactivity to GP, 11 MAbs, except for 1 MAb (from the A6-9 hybridoma) displaying an IgG2a type, were identified as IgM isotypes. Those 11 MAbs failed to recognize GP. However, the MAb from A6-9 recognized the mucin-like region of GP and remained reactive to the antigen at the lowest tested concentration (1.95 ng/mL). This result suggests that IgM-secreting hybridomas are predominantly generated by DNA vaccination. However, boosting with GP resulted in greater production of IgG-secreting hybridomas than GP DNA vaccination alone.
CONCLUSION
DNA vaccination may preferentially generate IgM-secreting hybridomas, but boosting with the protein antigen can reverse this propensity. Thus, this protein boosting approach may have implications for the production of IgG-specific hybridomas in the context of the DNA vaccination platform. In addition, the purified monoclonal IgG antibodies may be useful as therapeutic antibodies for controlling Ebola virus infection.
Keyword
MeSH Terms
-
Animals
Antibodies
Antibodies, Monoclonal
Antibody Formation
Blotting, Western
Clinical Coding*
DNA*
Ebolavirus*
Enzyme-Linked Immunosorbent Assay
Glycoproteins*
Hemorrhagic Fever, Ebola
Hybridomas*
Immunization*
Immunoglobulin G
Immunoglobulin M
Mice
Vaccination
Vaccines, DNA*
Antibodies
Antibodies, Monoclonal
DNA
Glycoproteins
Immunoglobulin G
Immunoglobulin M
Vaccines, DNA
Figure
Cited by 1 articles
-
Purification and characterization of monoclonal IgG antibodies recognizing Ebola virus glycoprotein
Baek-Sang Han, Ho-Young Jang, Trina Racine, Xiangguo Qiu, Jeong-Im Sin
Clin Exp Vaccine Res. 2018;7(2):119-128. doi: 10.7774/cevr.2018.7.2.119.
Reference
-
1. Roshania R, Mallow M, Dunbar N, et al. Successful implementation of a multicountry clinical surveillance and data collection system for Ebola virus disease in west Africa: findings and lessons learned. Glob Health Sci Pract. 2016; 4:394–409. PMID: 27688716.
Article2. Wilson JA, Hevey M, Bakken R, et al. Epitopes involved in antibody-mediated protection from Ebola virus. Science. 2000; 287:1664–1666. PMID: 10698744.
Article3. Parren PW, Geisbert TW, Maruyama T, Jahrling PB, Burton DR. Pre- and postexposure prophylaxis of Ebola virus infection in an animal model by passive transfer of a neutralizing human antibody. J Virol. 2002; 76:6408–6412. PMID: 12021376.
Article4. Leroy EM, Baize S, Volchkov VE, et al. Human asymptomatic Ebola infection and strong inflammatory response. Lancet. 2000; 355:2210–2215. PMID: 10881895.
Article5. Qiu X, Audet J, Wong G, et al. Successful treatment of ebola virus-infected cynomolgus macaques with monoclonal antibodies. Sci Transl Med. 2012; 4:138ra81.
Article6. Qiu X, Audet J, Wong G, et al. Sustained protection against Ebola virus infection following treatment of infected nonhuman primates with ZMAb. Sci Rep. 2013; 3:3365. PMID: 24284388.
Article7. Aihara H, Miyazaki J. Gene transfer into muscle by electroporation in vivo. Nat Biotechnol. 1998; 16:867–870. PMID: 9743122.
Article8. Rizzuto G, Cappelletti M, Maione D, et al. Efficient and regulated erythropoietin production by naked DNA injection and muscle electroporation. Proc Natl Acad Sci U S A. 1999; 96:6417–6422. PMID: 10339602.
Article9. Ahlen G, Soderholm J, Tjelle T, et al. In vivo electroporation enhances the immunogenicity of hepatitis C virus nonstructural 3/4A DNA by increased local DNA uptake, protein expression, inflammation, and infiltration of CD3+ T cells. J Immunol. 2007; 179:4741–4753. PMID: 17878373.
Article10. Lee IH, Park JB, Cheong M, Choi YS, Park D, Sin JI. Antitumor therapeutic and antimetastatic activity of electroporation-delivered human papillomavirus 16 E7 DNA vaccines: a possible mechanism for enhanced tumor control. DNA Cell Biol. 2011; 30:975–985. PMID: 21649506.
Article11. Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. 1975. J Immunol. 2005; 174:2453–2455. PMID: 15728446.12. Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press;1988.13. Krieg AM, Yi AK, Matson S, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995; 374:546–549. PMID: 7700380.
Article14. Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci U S A. 1996; 93:2879–2883. PMID: 8610135.
Article15. Sin JI, Bagarazzi M, Pachuk C, Weiner DB. DNA priming-protein boosting enhances both antigen-specific antibody and Th1-type cellular immune responses in a murine herpes simplex virus-2 gD vaccine model. DNA Cell Biol. 1999; 18:771–779. PMID: 10541436.
Article16. Sin JI, Ayyavoo V, Boyer J, Kim J, Ciccarelli RB, Weiner DB. Protective immune correlates can segregate by vaccine type in a murine herpes model system. Int Immunol. 1999; 11:1763–1773. PMID: 10545480.
Article17. Alvarez CP, Lasala F, Carrillo J, Muniz O, Corbi AL, Delgado R. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J Virol. 2002; 76:6841–6844. PMID: 12050398.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Purification and characterization of monoclonal IgG antibodies recognizing Ebola virus glycoprotein
- Effect of Anti - idiotype Antibody on Anti - DNA Antibody Production by Hybridoma Cells
- Production of Monoclonal Anti-idiotypic Antibody to Monoclonal Anti-DNA Antibody
- Characterization of Monoclonal Antibody Specific for Hepatitis C Virus E2 Envelope Protein
- Ebola outbreak in Western Africa 2014: what is going on with Ebola virus?