Clin Exp Vaccine Res.
2018 Jul;7(2):119-128. 10.7774/cevr.2018.7.2.119.
Purification and characterization of monoclonal IgG antibodies recognizing Ebola virus glycoprotein
- Affiliations
-
- 1BK21 Plus Graduate Program and Department of Microbiology, Kangwon National University School of Medicine, Chuncheon, Korea. jsin1964@hanmail.net
- 2Centre de Recherche en Infectiologie de l'Université Laval CHU de Québec-Université Laval, Québec, Canada.
- 3Department of Medical Microbiology University of Manitoba, Winnipeg, Canada.
- 4Special Pathogens Program, National Microbiology Laboratory, Public Health Agency of Canada, Winnipeg, Canada.
- KMID: 2418090
- DOI: http://doi.org/10.7774/cevr.2018.7.2.119
Abstract
- PURPOSE
The goal of this study was to purify and characterize Ebola virus glycoprotein (GP)-specific IgG antibodies from hybridoma clones.
MATERIALS AND METHODS
For hybridoma production, mice were injected by intramuscular-electroporation with GP DNA vaccines, and boosted with GP vaccines. The spleen cells were used for producing GP-specific hybridoma. Enzyme-linked immunosorbent assay, Western blot assay, flow cytometry, and virus-neutralizing assay were used to test the ability of monoclonal IgG antibodies to recognize GP and neutralize Ebola virus.
RESULTS
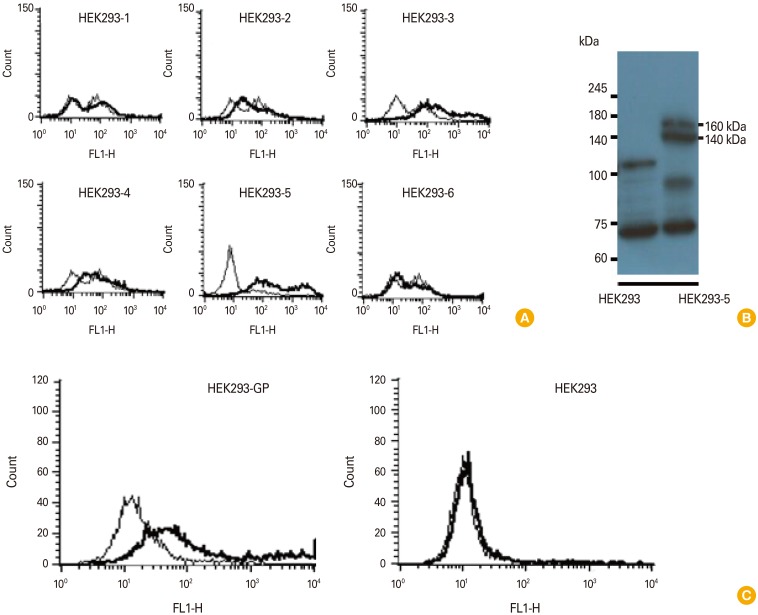
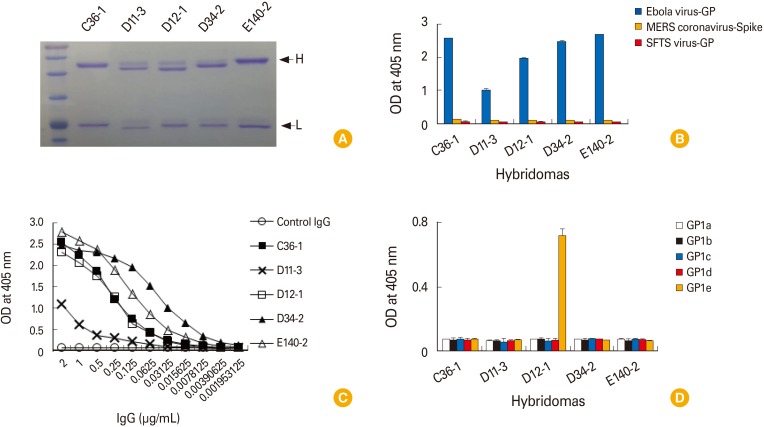
Twelve hybridomas, the cell supernatants of which displayed GP-binding activity by enzyme-linked immunosorbent assay and the presence of both IgG heavy and light chains by Western blot assay, were chosen as a possible IgG producer. Among these, five clones (C36-1, D11-3, D12-1, D34-2, and E140-2) were identified to secrete monoclonal IgG antibodies. When the monoclonal IgG antibodies from the 5 clones were tested for their antigen specificity, they recognized GP in an antigen-specific and IgG dose-dependent manner. They remained reactive to GP at the lowest tested concentrations (1.953-7.8 ng/mL). In particular, IgG antibodies from clones D11-3, D12-1, and E140-2 recognized the native forms of GP expressed on the cell surface. These antibodies were identified as IgG1, IgG2a, or IgG2b kappa types and appeared to recognize the native forms of GP, but not the denatured forms of GP, as determined by Western blot assay. Despite their GP-binding activity, none of the IgG antibodies neutralized Ebola virus infection in vitro, suggesting that these antibodies are unable to neutralize Ebola virus infection.
CONCLUSION
This study shows that the purified IgG antibodies from 5 clones (C36-1, D11-3, D12-1, D34-2, and E140-2) possess GP-binding activity but not Ebola virus-neutralizing activity.
MeSH Terms
-
Animals
Antibodies*
Antibody Formation
Blotting, Western
Clone Cells
Ebolavirus*
Enzyme-Linked Immunosorbent Assay
Flow Cytometry
Glycoproteins*
Hemorrhagic Fever, Ebola
Hybridomas
Immunoglobulin G*
In Vitro Techniques
Mice
Sensitivity and Specificity
Spleen
Vaccines
Vaccines, DNA
Antibodies
Glycoproteins
Immunoglobulin G
Vaccines
Vaccines, DNA
Figure
Reference
-
1. Roshania R, Mallow M, Dunbar N, et al. Successful implementation of a multicountry clinical surveillance and data collection system for Ebola virus disease in West Africa: findings and lessons learned. Glob Health Sci Pract. 2016; 4:394–409. PMID: 27688716.
Article2. Brooks GF, Carroll KC, Butel JS, Morse SA, Mietzner TA. Jawetz, Melnick and Adelberg's medical microbiology. New York: McGraw-Hill;2013. p. 571–573.3. Na W, Park N, Yeom M, Song D. Ebola outbreak in Western Africa 2014: what is going on with Ebola virus? Clin Exp Vaccine Res. 2015; 4:17–22. PMID: 25648530.
Article4. Alvarez CP, Lasala F, Carrillo J, Muniz O, Corbi AL, Delgado R. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J Virol. 2002; 76:6841–6844. PMID: 12050398.5. Ito H, Watanabe S, Sanchez A, Whitt MA, Kawaoka Y. Mutational analysis of the putative fusion domain of Ebola virus glycoprotein. J Virol. 1999; 73:8907–8912. PMID: 10482652.
Article6. Qiu X, Audet J, Wong G, et al. Successful treatment of ebola virus-infected cynomolgus macaques with monoclonal antibodies. Sci Transl Med. 2012; 4:138ra81.
Article7. Qiu X, Audet J, Wong G, et al. Sustained protection against Ebola virus infection following treatment of infected nonhuman primates with ZMAb. Sci Rep. 2013; 3:3365. PMID: 24284388.
Article8. PREVAIL II Writing Group. Multi-National PREVAIL II Study Team. Davey RT Jr, et al. A randomized, controlled trial of ZMapp for Ebola virus infection. N Engl J Med. 2016; 375:1448–1456. PMID: 27732819.
Article9. Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975; 256:495–497. PMID: 1172191.
Article10. Lee SH, Han BS, Choe J, Sin JI. Preferential production of IgM-secreting hybridomas by immunization with DNA vaccines coding for Ebola virus glycoprotein: use of protein boosting for IgG-secreting hybridoma production. Clin Exp Vaccine Res. 2017; 6:135–145. PMID: 28775978.
Article11. Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press;1988. p. 222–223.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Preferential production of IgM-secreting hybridomas by immunization with DNA vaccines coding for Ebola virus glycoprotein: use of protein boosting for IgG-secreting hybridoma production
- Characterization of monoclonal antibodies against human cytomegalovirus(HCMV) glycoprotein
- Generation and Characterization of Anti - Human CTLA - 4 Monoclonal Antibodies
- Production and characterization of monoclonal antibodies to perchloric acid soluble antigen of M.tuberculosis and its clinical application
- Ebola outbreak in Western Africa 2014: what is going on with Ebola virus?