Lab Anim Res.
2017 Sep;33(3):264-269. 10.5625/lar.2017.33.3.264.
Dose-dependent effects of busulfan on dog testes in preparation for spermatogonial stem cell transplantation
- Affiliations
-
- 1Animal Biotechnology Division, National Institute of Animal Science, RDA, Korea.
- 2Department of Stem Cell and Regenerative Biology, Konkuk University, Seoul, Korea.
- 3Department of Food Bioscience, College of Biomedical & Health Science, Konkuk University, Chung-ju, Korea.
- 4Research Group of Nutraceuticals for Metabolic Syndrome, Korea Food Research Institute, Seongnam, Korea. vetian@kfri.re.kr
- 5Department of Beef & Dairy Science, Korea National College of Agriculture and Fisheries, Jeonju, Korea. leewy81@korea.kr
- KMID: 2391101
- DOI: http://doi.org/10.5625/lar.2017.33.3.264
Abstract
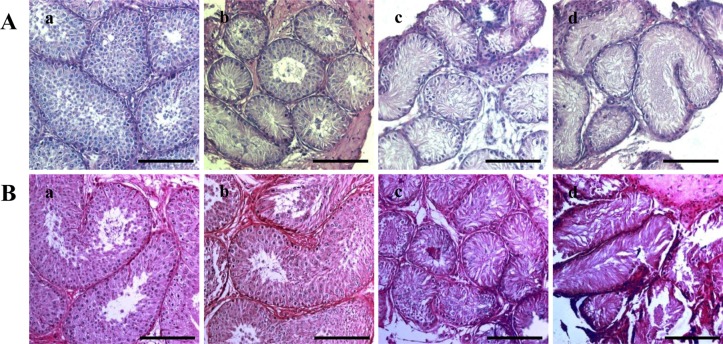
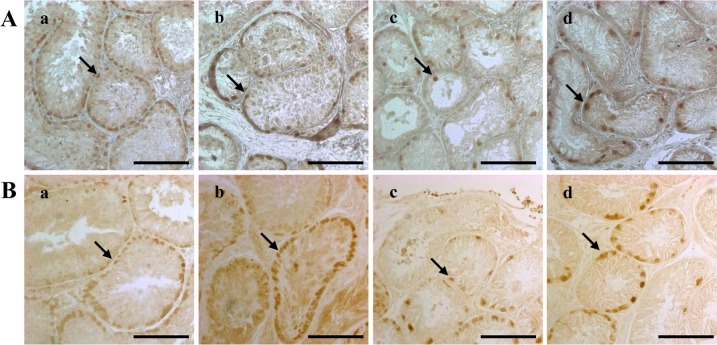
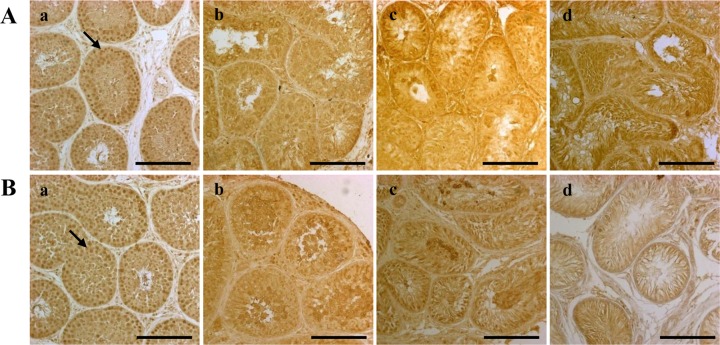
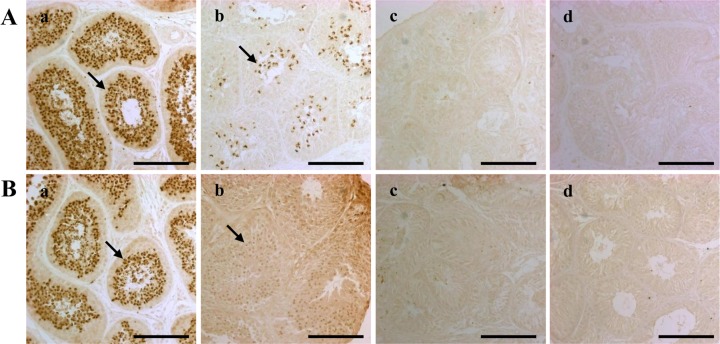
- Successful male germ cell transplantation requires depletion of the host germ cells to allow efficient colonization of the donor spermatogonial stem cells. Although a sterilizing drug, busulfan, is commonly used for the preparation of recipient models before transplantation, the optimal dose of this drug has not yet been defined in dogs. In this study, 1-year-old mongrel dogs were intravenously injected with three different concentrations of busulfan (10, 15, or 17.5 mg/kg). Four weeks after busulfan treatment, no fully matured spermatozoa were detected in any of the busulfan-treated groups. However, small numbers of PGP9.5-positive spermatogonia were detected in all treatment groups, although no synaptonemal complex protein-3-positive spermatocytes were detected. Of note, acrosin-positive spermatids were not detected in the dogs treated with 15 or 17.5 mg/kg busulfan, but were detected in the other group. Eight weeks after busulfan treatment, the dogs treated with 10 mg/kg busulfan fully recovered, but those in the other groups did not. PGP9.5-positive spermatogonia were detected in the 10 mg/kg group, and at a similar level as in the control group, but these cells were rarely detected in the 15 and 17.5 mg/kg groups. These results suggest that a dose of 15-17.5 mg/kg is optimal for ablative treatment with busulfan to prepare the recipient dogs for male germ cell transplantation. At least eight weeks should be allowed for recovery. The results of this study might facilitate the production of recipient dogs for male germ cell transplantation and can also contribute to studies on chemotherapy.
MeSH Terms
Figure
Reference
-
1. Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A. 1994; 91(24):11298–11302. PMID: 7972053.
Article2. Ogawa T, Dobrinski I, Brinster RL. Recipient preparation is critical for spermatogonial transplantation in the rat. Tissue Cell. 1999; 31(5):461–472. PMID: 10612257.
Article3. Jahnukainen K, Ehmcke J, Quader MA, Saiful Huq M, Epperly MW, Hergenrother S, Nurmio M, Schlatt S. Testicular recovery after irradiation differs in prepubertal and pubertal non-human primates, and can be enhanced by autologous germ cell transplantation. Hum Reprod. 2011; 26(8):1945–1954. PMID: 21613315.
Article4. Shinohara T, Avarbock MR, Brinster RL. Functional analysis of spermatogonial stem cells in Steel and cryptorchid infertile mouse models. Dev Biol. 2000; 220(2):401–411. PMID: 10753526.
Article5. Jegou B, Peake RA, Irby DC, de Kretser DM. Effects of the induction of experimental cryptorchidism and subsequent orchidopexy on testicular function in immature rats. Biol Reprod. 1984; 30(1):179–187. PMID: 6141812.6. Bucci LR, Meistrich ML. Effects of busulfan on murine spermatogenesis: cytotoxicity, sterility, sperm abnormalities, and dominant lethal mutations. Mutat Res. 1987; 176(2):259–268. PMID: 3807936.
Article7. Wang DZ, Zhou XH, Yuan YL, Zheng XM. Optimal dose of busulfan for depleting testicular germ cells of recipient mice before spermatogonial transplantation. Asian J Androl. 2010; 12(2):263–270. PMID: 20010847.
Article8. Kim JH, Jung-Ha HS, Lee HT, Chung KS. Development of a positive method for male stem cell-mediated gene transfer in mouse and pig. Mol Reprod Dev. 1997; 46(4):515–526. PMID: 9094099.
Article9. Hermann BP, Sukhwani M, Winkler F, Pascarella JN, Peters KA, Sheng Y, Valli H, Rodriguez M, Ezzelarab M, Dargo G, Peterson K, Masterson K, Ramsey C, Ward T, Lienesch M, Volk A, Cooper DK, Thomson AW, Kiss JE, Penedo MC, Schatten GP, Mitalipov S, Orwig KE. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell. 2012; 11(5):715–726. PMID: 23122294.
Article10. Deeg HJ, Schuler US, Shulman H, Ehrsam M, Renner U, Yu C, Storb R, Ehninger G. Myeloablation by intravenous busulfan and hematopoietic reconstitution with autologous marrow in a canine model. Biol Blood Marrow Transplant. 1999; 5(5):316–321. PMID: 10534062.
Article11. Lee WY, Lee R, Park HJ, Do JT, Park C, Kim JH, Jhun H, Lee JH, Hur T, Song H. Characterization of male germ cell markers in canine testis. Anim Reprod Sci. 2017; 182:1–8. See comment in PubMed Commons below. PMID: 28465081.
Article12. Hill JR, Dobrinski I. Male germ cell transplantation in livestock. Reprod Fertil Dev. 2006; 18(1-2):13–18. PMID: 16478598.
Article13. Brinster CJ, Ryu BY, Avarbock MR, Karagenc L, Brinster RL, Orwig KE. Restoration of fertility by germ cell transplantation requires effective recipient preparation. Biol Reprod. 2003; 69(2):412–420. PMID: 12672656.14. Hassan M, Oberg G, Björkholm M, Wallin I, Lindgren M. Influence of prophylactic anticonvulsant therapy on high-dose busulphan kinetics. Cancer Chemother Pharmacol. 1993; 33(3):181–186. PMID: 8269597.
Article15. Boettger-Tong HL, Johnston DS, Russell LD, Griswold MD, Bishop CE. Juvenile spermatogonial depletion (jsd) mutant seminiferous tubules are capable of supporting transplanted spermatogenesis. Biol Reprod. 2000; 63(4):1185–1191. PMID: 10993844.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Establishment of a surgically induced cryptorchidism canine recipient model for spermatogonial stem cell transplantation
- Transplantation of Autologous Bone Marrow Mesenchymal Stem Cells into the Testes of Infertile Male Rats and New Germ Cell Formation
- Targeted busulfan and fludarabine-based conditioning for bone marrow transplantation in chronic granulomatous disease
- The Effects of Muscle Relaxants on Histamine Contraction Isolated Trachesl Preparation of Mongrel Dogs in Vitro
- Safety and Pharmacokinetics of Intravenous Busulfan as Conditioning prior to Allogeneic Stem Cell Transplantation